COAL TREATMENT
Cleaning coal prior to combustion can significantly reduce the levels of sulfur emissions from a power plant, as well as reduce the amount of ash and slag produced. Physical cleaning, as outlined earlier, can have a beneficial effect on plant performance and maintenance schedules. It has been estimated that boiler availability improves by 1% for every 1% reduction in ash content. This may be cost effective, depending on the quality of coal being treated. However, more complex cleaning procedures have not so far proved economical.
One disadvantage of coal cleaning is that it leads to a loss of between 2% and 15% of the coal with the coal waste. It may be possible to burn this coal waste in a fluidized bed combustion plant, thus reducing overall losses.
LOW NITROGEN OXIDE COMBUSTION STRATEGIES
When coal is burned, oxides of nitrogen, including nitrogen dioxide, nitrogen oxide, and nitrous oxide, can be generated. Together these are generally termed nitrogen oxide, or NOx. Nitrogen oxide is the product of the oxidation of the nitrogen in air with oxygen, also in the air, at the high temperatures found during coal combustion. The amount produced depends on two factors: the temperature at which the combustion takes place and the amount of oxygen available. If the amount of oxygen is restricted, then it will preferentially react with carbon rather than nitrogen, thereby reducing the production of nitrogen oxide. The rate of the reaction is also affected by the temperature; the lower the temperature the slower the reaction and the less reaction product. Both of these conditions can be exploited in a coal-fired power plant to maintain as low a level as possible in the flue gases.
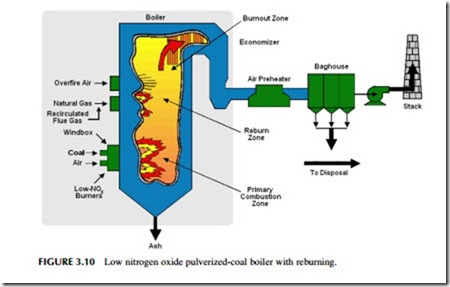
One of the key strategies is to restrict the amount of oxygen available for combustion in the hottest part of the furnace (Figure 3.10). In a PC power plant, powered coal and air are generally admitted into the combustion chamber
together in a continuous flow. To keep nitrogen oxide production low, some of the air needed to burn the coal completely is prevented from entering the initial combustion region with the coal; instead it is delayed briefly, being admitted to this primary combustion region after some of the combustion has been com- pleted. This staged combustion procedure (as it is commonly known) can reduce the level of nitrogen oxide produced by 30–55%.
The initial combustion zone is normally the hottest region in the furnace. As the combustion gases leave this zone they start to cool. At this stage further air can be admitted (if combustion of the pulverized coal is still incomplete) to allow the combustion of the fuel to be completed, but at a lower temperature where the production of nitrogen oxide is reduced. The air admitted at this stage in the furnace is called overfire-air. When used in conjunction with controlled air admission in the main combustion zone, the use of overfire air can lead to a reduction in nitrogen oxide levels of 40–60%.
A third strategy that can reduce the nitrogen oxide levels even further is called reburning. This simply means that more coal, or natural gas, is introduced into the combustion gases after they have left the combustion zone. By this time gaseous oxygen levels in the flue gases will have been greatly reduced so that the new fuel will effectively steal oxygen from nitrogen oxide to combust. The effect is to remove some of the oxides of nitrogen that have been formed. Over- all reductions of up to 70% can be achieved when all three strategies are applied. Low nitrogen oxide burners, overfire air, and reburning are all strategies that can be introduced into existing coal-fired power plants that do not have them, as well as being incorporated into new plants.