cells and Batteries
As mentioned in the previous unit, a cell contains a positive and a negative electrode separated by an electrolytic solution. A battery is a combination of two or more cells. There are two basic types of cells. Cells that
cannot be recharged are called primary cells. Cells that can be recharged are called secondary cells.
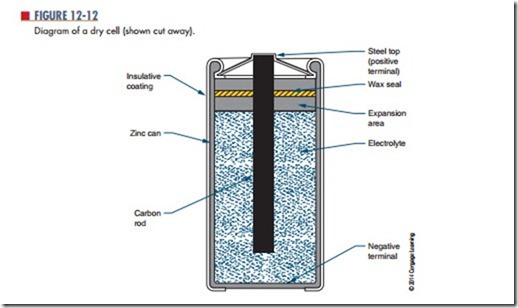
An example of a primary cell is a Leclanche cell, also called a dry cell (Figure 12-12). This type of cell is not actually dry. It contains a moist paste as the electrolyte. A seal prevents the paste from leaking out when the cell is turned sideways or upside down. The electrolyte in a dry cell is a solution of ammonium chloride and manganese dioxide. The electrolyte dis- solves the zinc electrode (the case of the cell), leaving an excess of electrons with the zinc. As the current is removed from the cell, the zinc, ammonium chloride, and manganese dioxide produce manganese dioxide, water, ammonia, and zinc chloride. The carbon rod (center electrode) gives up the extra electrons that ac- cumulate on the zinc electrode. This type of cell pro- duces as much as 1.75 to 1.8 volts when new. A typical Leclanche cell has an energy density of approximately 120 watt-hours per pound. As the cell is used, the chemical action decreases, and eventually the result- ing current ceases. If the cell is not used, the electro- lytic paste eventually dries out. The cell has a shelf life of about two years. The output voltage of this type of cell is determined entirely by the materials used for the electrolyte and the electrodes. The AAA cell, AA cell, C cell, and D cell (Figure 12-13) are all constructed of the same materials and therefore produce the same
voltage. It should be noted that although the Leclanche cell is frequently referred to as a carbon-zinc (or zinc carbon) cell, the carbon does not take any part in the chemical reaction that produces electricity.
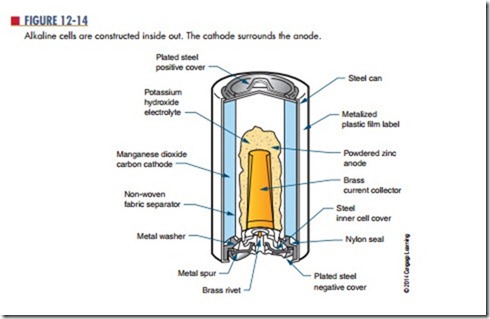
The alkaline cell is named because of the highly caustic base, potassium hydroxide (KOH), used as the electrolyte. The design of an alkaline cell on the outside is very similar to that of a carbon-zinc cell. However, the inside of the alkaline cell is significantly different (Figure 12-14). Alkaline cells have an
open-circuit rating of approximately 1.52 volts, and an energy density of about 45 watt-hours per pound. The alkaline cell performs much better over temperature extremes than carbon-zinc cells. Alkaline cells per- form best where moderate to high currents are drawn over extended periods of time.
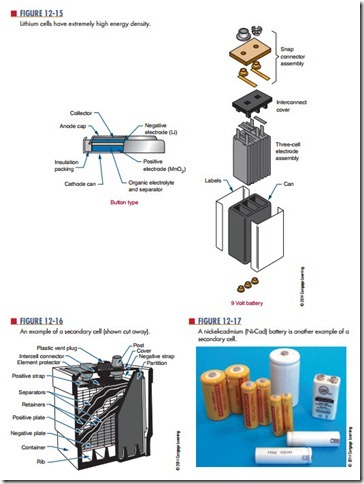
lithium cells (Figure 12-15) have overcome the inherent properties associated with lithium. Lithium is extremely reactive with water. Lithium cell formation uses lithium, manganese dioxide (MnO2), and a lithium perchlorate (LiCIO4) in an organic solvent
(water cannot be used). The output of a lithium cell is approximately 3 volts. Lithium cells are very efficient, with energy densities of about 90 watt-hours per pound. The greatest benefit of lithium cells is their extremely long shelf life of 5 to 10 years.
A secondary cell is a cell that can be recharged by applying a reverse voltage. An example is the lead-acid battery used in automobiles (Figure 12-16). It is made of six 2-volt secondary cells connected in series. Each cell has a positive electrode of lead peroxide (PbO2) and a negative electrode of spongy lead (Pb). The electrodes are separated by plastic or rubber and immersed in an electrolytic solution of sulfuric acid (H2SO4) and dis- tilled water (H2O). As the cell is discharged, the sulfuric acid combines with the lead sulfate, and the electrolyte converts to water. Recharging the cell involves applying a source of DC voltage greater than that produced by the cell. As the current flows through the cell, it changes the electrode back to lead peroxide and spongy lead and converts the electrolyte back to sulfuric acid and water. This type of cell is also referred to as a wet cell.
Another type of secondary cell is the nickel cadmium (ni-cad) cell (Figure 12-17). This is a dry cell that can be recharged many times and can hold its charge for long periods of time. It consists of a positive and a negative electrode, a separator, an electrolyte, and a package. The electrodes are comprised of a de- posit of powdered nickel on a nickel wire screen, which is coated with a nickel salt solution for the positive electrode and a cadmium salt solution for a negative electrode. The separator is made of an absorbent insulating material. The electrolyte is potassium hydrox- ide. A steel can forms the package and is sealed tightly. A typical voltage from this type of cell is 1.2 volts.
The ability of a battery to deliver power continuously is expressed in ampere-hours. A battery rated at 100 ampere-hours can continuously supply any of the following: 100 amperes for 1 hour (100 3 1 5 100 ampere-hours), 10 amperes for 10 hours (10 3 10 5 100 ampere-hours), or 1 ampere for 100 hours (1 3 100 5 100 ampere-hours).
Questions
1. What are the components of a cell?
2. What are the two basic types of cells?
3. What is the major difference between the two
types of cells?
4. List some examples of a primary cell.
5.List some examples of a secondary cell.