Color Perception
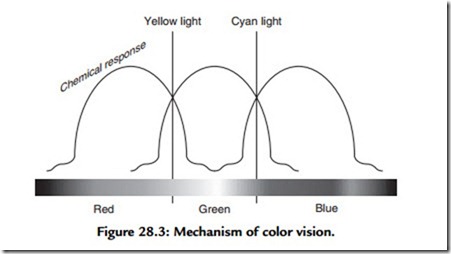
Sir Isaac Newton discovered that sunlight passing through a prism breaks into the band of multicolored light, which we now call a spectrum. We perceive seven distinct bands in the spectrum: red, orange, yellow, green, blue, indigo, and violet. We see these bands distinctly because each represents a particular band of wavelengths. The objects we perceive as colored are perceived thus because they too reflect a particular range of wavelengths. For instance, a daffodil looks yellow because it reflects predominantly wavelengths in the region 570 nm. We can experience wavelengths of different color because the cone cells, in the retina at the back of the eye, contain three photosensitive chemicals, each of which is sensitive in three broad areas of the light spectrum. It is easiest to think of this in terms of three separate but overlapping photochemical processes: a low-frequency (long-wavelength) RED process, a medium-frequency GREEN process, and a high-frequency BLUE process. (Electronic engineers might prefer to think of this as three, shallow-slope band-pass filters!) When light of a particular frequency falls on the retina, the action of the light reacts selectively with this frequency- discriminating mechanism. When we perceive a red object we are experiencing a high level of activity in our long wavelength (low-frequency) process and low levels in our other two. A blue object stimulates the short wavelength or high-frequency process and so on. When we perceive an object with an intermediate color, say the yellow of the egg yoke, we experience a mixture of two chemical processes caused by the overlapping nature of each of the frequency-selective mechanisms. In this case, the yellow light from the egg causes stimulation in both the long wavelength RED process and the medium- wavelength GREEN process (Figure 28.3). Because human beings possess three separate color vision processes we are classified as trichromats. People afflicted with color blindness usually lack one of the three chemical responses in the normal eye; they are known as dichromats, although a few rare individuals are true monochromats. What has not yet been discovered, among people or other animals, is a more-than-three color perception system. This is fortunate for the engineers who developed color television!
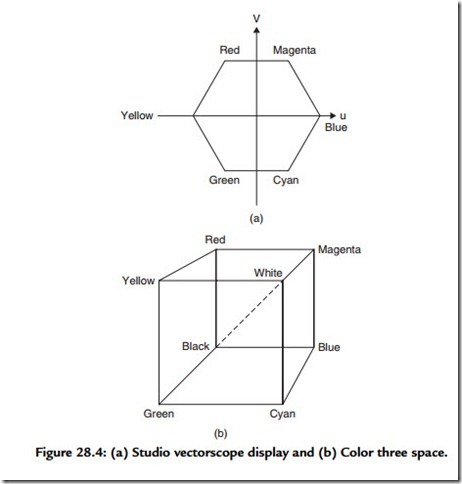
The fact that our cone cells only contain three chemicals is why we may be fooled into experiencing the whole gamut of colors with the combination of only three so-called primary colors. The television primaries of red, green, and blue were chosen because each stimulates only one of the photosensitive chemicals found in the cone cells. The great television swindle is that we can, for instance, be duped into believing we are seeingyellow by activating both the red and green tube elements simultaneously—just as would a pure yellow source. Similarly we may be hoodwinked into seeing light blue cyan with the simultaneous activation of green and blue. We can also be made to experience paradoxical colors such as magenta by combining red and blue, a feat that no pure light source could ever do! This last fact demonstrates that our color perception system effectively “wraps around,” mapping the linear spectrum of electromagnetic frequencies into a color circle, or a color space. And it is in this way that we usually view the science of color perception: we can regard all visual sense as taking place within a color three space. A television studio vectorscope allows us to view color three space end on, so it looks like a hexagon [Figure 28.4(a)]. Note that each color appears at a different angle, like the numbers on a
clock face. Hue is the term used in image processing and television to describe a color’s precise location on this locus. Saturation is the term used to describe the amount a pure color is “diluted” by white light. The dotted axis shown in Figure 28.4(b) is the axis of pure luminance. The more a particular shade moves towards this axis from a position on the boundary of the cube, the more a color is said to be desaturated.