Gases
The velocity of gases can be measured in the same ways as are used for
liquids, but a few specialized methods are added. The anemometer is used for wind-speed measurements in meteorology, and consists of a set of cup- shaped vanes that rotate in a horizontal plane (Figure 6.28). The speed of rotation is proportional to wind speed, and is most conveniently measured by using a tachometer or a rotary digitizer connected to electronic circuits. The application of electronic methods to anemometers has increased the precision of these sensors in comparison to the older types in which the vanes were coupled to an indicator by a revolving cable.
Hot-wire methods are applied to gas movement as well as to gas pressure (see Pirani gauge, Chapter 1). A wire that is exposed to the moving gas is heated by the passage of a current. The temperature that the wire reaches depends on the cooling effect of the moving gas, so that the resistance of the wire can be used as a measure of its temperature and so of the gas velocity. The relationship between wire resistance and gas flow is not a simple one, and a sensor of this type needs to be calibrated against a more fundamental measurement. In addition, the calibration will need to be repeated if the composition of the gas is changed at any time.
The application of electronics to car engines has created a requirement for a number of gas sensors, particularly flow, temperature and composition sensors. Gas flow can very often be sensed by simple vane-deflection methods, because linearity is seldom an overriding requirement. Gas com- position usually means exhaust gas oxygen content, which is a critical factor that measures the efficiency of combustion. The oxygen content of exhaust gas is sensed by using metal oxide ‘cells’, for which zirconium oxide or titanium oxide are the most suitable types. When either of these oxides is sandwiched between platinum electrodes, an EMF will be generated whose size depends on the availability of oxygen ions surrounding the sensor. By allowing the hot exhaust gas to pass over the cell, then, the amount of oxygen in the exhaust can be sensed, and so the efficiency of combustion assessed. The important feature of such a sensor for use in cars is that the change in combustion conditions from lean (too much oxygen) to rich (too much fuel) causes a very large change of oxygen content (for example, a ratio of 1014) so that the output of the sensor can change very sharply in the range 20 mV to 1 V.
In general, sensors for gas composition rely either on cells whose output is an EMF, or on the effect of the absorption of the gas on a quartz crystal that is part of an oscillating circuit. These types of transducers are very specialized, and many of the cell types make use of the catalytic action of platinum or palladium in thin films. All such sensors are very susceptible to ‘poisoning’ by unwanted contaminants in the gases, and particularly metallic contaminants such as lead.
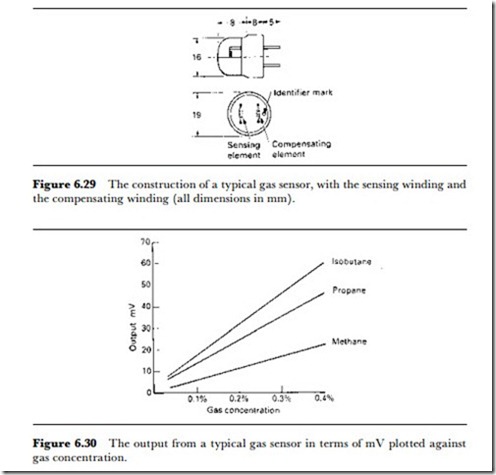
A typical gas composition unit for flammable and other gases is the RS Components gas sensor NAP11A. This uses a platinum spiral sensor and a compensating winding in a bridge arrangement, both heated, but only one is exposed to the gas. Following the age-old principle of the Davy lamp, the exposed spiral is enclosed within two layers of wire mesh, preventing explosion of mixtures that contain oxygen mixed with the flammable gases. The outline of the sensor itself is illustrated in Figure 6.29 and its performance with some gases in Figure 6.30. A typical applications circuit, courtesy of RS components, is shown in Figure 6.31. Data sheets can be downloaded from the RS Web site.
Some points to consider in the use of such sensors are that silicone products (greases, oils and polishes) will ruin the performance of the gas detector, and long-term exposure to salty atmospheres will also affect the calibration. These units are intended for detecting small concentrations of gases such as might indicate a dangerous build-up, and must not be used with concentrated gases. Their use in gas alarms must be well thought out,
because some flammable gases are less dense than air, and will be found mainly at the top of an enclosed space. Others are denser than air and will be found at the bottom of a space – cellars and ships’ bilges are particularly hazardous in this respect.
• A more recent development is the use of metal-oxide gas detectors, particularly the use of tin dioxide. This has led to work on sensors that will detect carbon monoxide in the presence of unburned fuel residues, sensors that will detect unburned fuel despite the presence of carbon monoxide, sensors for hydrogen in the presence of hydrocarbons, and sensors for nitrogen oxides. All of the applications are of major importance in detecting and possibly controlling car exhaust emissions.