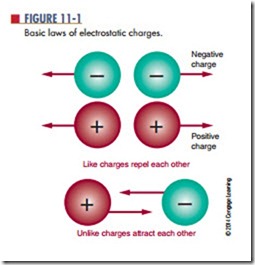
Two electrons together or two protons together represent “like” charges. Like charges resist being brought together and instead move away from each other. This movement is called repelling. This is the first law of electrostatic charges: Like charges repel each other (Figure 11-1). According to the second law of electro- static charges: Unlike charges attract each other.
The negative electrons are drawn toward the positive protons in the nucleus of an atom. This attractive force is balanced by the centrifugal force caused by the electron’s rotation about the nucleus. As a result, the electrons re- main in orbit and are not drawn into the nucleus.
The amount of attracting or repelling force that acts between two electrically charged bodies depend on two factors: their charge and the distance between them.
Single electrons have a charge too small for practical use. The unit adopted for measuring charges is the coulomb (C), named for Charles Coulomb. The electrical
charge (Q) carried by 6,240,000,000,000,000,000 electrons (six quintillion two hundred forty quadrillion, or 6.24 3 1018) represents one coulomb.
Electrical charges are created by the displacement of electrons. When there is an excess of electrons at one point and a deficiency of electrons at another point, a difference of potential exists between the two points. When a difference of potential exists between two charged bodies connected by a conductor, electrons will flow along the conductor. This flow of electrons is called current.
Questions
1. What are the two laws of electrostatic charges?
2. What does an electrical charge represent?
3. Define coulomb.
4. How many electrons are in 3 coulombs?
5. What is the flow of electrons called?