The measurement of temperature
A change in temperature of a substance can often result in a change in one or more of its physical properties. Thus, although temperature cannot be measured directly, its effects can be measured. Some properties of substances used to determine changes in temperature include changes in dimensions, electrical resistance, state, type and volume of radiation and colour.
Temperature measuring devices available are many and varied. Those described in this chapter are those most often used in science and industry.
At the end of this chapter you should be able to:
• describe the construction, principle of operation and practical applications of the following temperature measuring devices:
(a) liquid-in-glass thermometer (including ad- vantages of mercury, and sources of error)
(b) thermocouples (including advantages and sources of error)
(c) resistance thermometer (including limita- tions and advantages of platinum coil)
(d) thermistors
(e) pyrometers (total radiation and optical types, including advantages and disadvantages)
• describe the principle of operation of
(a) temperature indicating paints and crayons
(b) bimetallic thermometers
(c) mercury-in-steel thermometer
(d) gas thermometer
• select the appropriate temperature measuring device for a particular application.
Liquid-in-glass thermometer
A liquid-in-glass thermometer uses the expansion of a liquid with increase in temperature as its principle of operation.
Construction
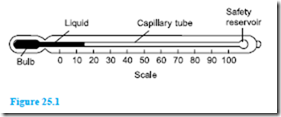
A typical liquid-in-glass thermometer is shown in Figure 25.1 and consists of a sealed stem of uniform small-bore tubing, called a capillary tube, made of glass, with a cylindrical glass bulb formed at one end. The bulb and part of the stem are filled with a liquid such as mercury or alcohol and the remaining part of the tube is evacuated. A temperature scale is formed by etching graduations on the stem. A safety reservoir is usually provided, into which the liquid can expand without bursting the glass if the temperature is raised beyond the upper limit of the scale.
Principle of operation
The operation of a liquid-in-glass thermometer depends on the liquid expanding with increase in temperature and contracting with decrease in temperature. The position of the end of the column of liquid in the tube is a measure of the temperature of the liquid in thebulb – shown as 15°C in Figure 25.1, which is about room temperature. Two fixed points are needed to calibrate the thermometer, with the interval between these points being divided into ‘degrees’. In the first thermometer, made by Celsius, the fixed points chosen were the temperature of melting ice (0°C) and that of boiling water at standard atmospheric pressure (100°C), in each case the blank stem being marked at the liquid level. The distance between these two points, called the fundamental interval, was divided into 100 equal parts, each equivalent to 1°C, thus forming the scale.
The clinical thermometer, with a limited scale around body temperature, the maximum and/or minimum thermometer, recording the maximum day temperature and minimum night temperature, and the Beckman thermometer, which is used only in accurate measurement of temperature change, and has no fixed points, are particular types of liquid-in-glass thermometer which all operate on the same principle.
Advantages
The liquid-in-glass thermometer is simple in construc- tion, relatively inexpensive, easy to use and portable, and is the most widely used method of temperature measurement having industrial, chemical, clinical and meteorological applications.
Disadvantages
Liquid-in-glass thermometers tend to be fragile and hence easily broken, can only be used where the liquid column is visible, cannot be used for surface temperature measurements, cannot be read from a distance and are unsuitable for high temperature measurements.
Advantages of mercury
The use of mercury in a thermometer has many advantages, for mercury:
(i) is clearly visible,
(ii) has a fairly uniform rate of expansion,
(iii) is readily obtainable in the pure state,
(iv) does not ‘wet’ the glass,
(v) is a good conductor of heat.
Mercury has a freezing point of –39°C and can- not be used in a thermometer below this temperature. Its boiling point is 357°C but before this temperature is reached some distillation of the mercury occurs if the space above the mercury is a vacuum. To prevent this, and to extend the upper temperature limits to over 500°C, an inert gas such as nitrogen under pressure is used to fill the remainder of the capillary tube. Alcohol, often dyed red to be seen in the capillary tube, is considerably cheaper than mercury and has a freezing point of –113°C, which is considerably lower than for mercury. However it has a low boiling point at about 79°C.
Errors
Typical errors in liquid-in-glass thermometers may oc- cur due to:
(i) the slow cooling rate of glass
(ii) incorrect positioning of the thermometer
(iii) a delay in the thermometer becoming steady (i.e. slow response time)
(iv) non-uniformity of the bore of the capillary tube, which means that equal intervals marked on the stem do not correspond to equal temperature intervals.
Thermocouples
Thermocouples use the e.m.f. set up when the junction of two dissimilar metals is heated.
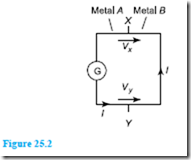
Principle of operation At the junction between two different metals, say, cop- per and constantan, there exists a difference in electrical potential, which varies with the temperature of the junction. This is known as the ‘thermo-electric effect’. If the circuit is completed with a second junction at a different temperature, a current will flow round the circuit. This principle is used in the thermocouple. Two different metal conductors having their ends twisted together are shown in Figure 25.2. If the two junctions are at different temperatures, a current I flows round the circuit.
The deflection on the galvanometer G depends on the difference in temperature between junctions X and Y and is caused by the difference between voltages Vx and Vy. The higher temperature junction is usually called the ‘hot junction’ and the lower temperature junction the ‘cold junction’. If the cold junction is kept at a constant known temperature, the galvanometer can be calibrated to indicate the temperature of the hot junction directly. The cold junction is then known as the reference junction.
In many instrumentation situations, the measuring instrument needs to be located far from the point at which the measurements are to be made. Extension leads are then used, usually made of the same material as the thermocouple but of smaller gauge. The reference junction is then effectively moved to their ends. The thermocouple is used by positioning the hot junction where the temperature is required. The meter will indicate the temperature of the hot junction only if the reference junction is at 0°C for:
(temperature of hot junction) = (temperature of the cold junction) + (temperature difference)
In a laboratory the reference junction is often placed in melting ice, but in industry it is often positioned in a thermostatically controlled oven or buried under- ground where the temperature is constant.
Construction
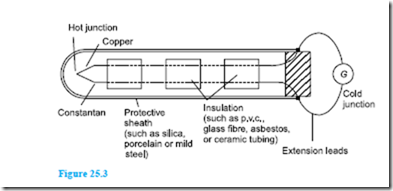
Thermocouple junctions are made by twisting together the ends of two wires of dissimilar metals before welding them. The construction of a typical copper- constantan thermocouple for industrial use is shown in Figure 25.3. Apart from the actual junction the two conductors used must be insulated electrically from each other with appropriate insulation and is shown in Figure 25.3 as twin-holed tubing. The wires and insulation are usually inserted into a sheath for protection from environments in which they might be damaged or corroded.
Applications
A copper-constantan thermocouple can measure temperature from –250°C up to about 400°C, and is used typically with boiler flue gases, food processing and with sub-zero temperature measurement. An iron- constantan thermocouple can measure temperature from –200°C to about 850°C, and is used typically in paper and pulp mills, re-heat and annealing furnaces and in chemical reactors. A chromel-alumel thermo- couple can measure temperatures from –200°C to about 1100°C and is used typically with blast furnace gases, brick kilns and in glass manufacture.
For the measurement of temperatures above 1100°C radiation pyrometers are normally used. However, thermocouples are available made of platinum- platinum/rhodium, capable of measuring temperatures up to 1400°C, or tungsten-molybdenum which can measure up to 2600°C.
Advantages
A thermocouple:
(i) has a very simple, relatively inexpensive con- struction
(ii) can be made very small and compact
(iii) is robust
(iv) is easily replaced if damaged
(v) has a small response time
(vi) can be used at a distance from the actual mea- suring instrument and is thus ideal for use with automatic and remote-control systems.
Sources of error
Sources of error in the thermocouple, which are difficult to overcome, include:
(i) voltage drops in leads and junctions
(ii) possible variations in the temperature of the cold junction
(iii) stray thermoelectric effects, which are caused by the addition of further metals into the ‘ideal’ two-metal thermocouple circuit.
Additional leads are frequently necessary for extension leads or voltmeter terminal connections.
A thermocouple may be used with a battery- or mains-operated electronic thermometer instead of a millivoltmeter. These devices amplify the small e.m.f.’s from the thermocouple before feeding them to a multi-range voltmeter calibrated directly with tem- perature scales. These devices have great accuracy and are almost unaffected by voltage drops in the leads and junctions.
Problem 1. A chromel-alumel thermocouple generates an e.m.f. of 5 mV. Determine the temperature of the hot junction if the cold junction is at a temperature of 15°C and the sensitivity of the thermocouple is 0.04 mV/°C.
Practise Exercise 137 Further problem on the thermocouple
1. A platinum–platinum/rhodium thermocouple generates an e.m.f. of 7.5 mV. If the cold junction is at a temperature of 20°C, deter- mine the temperature of the hot junction. Assume the sensitivity of the thermocouple to be 6 μV/°C [ 1270°C ]
Resistance thermometers
Resistance thermometers use the change in electrical resistance caused by temperature change.
Construction
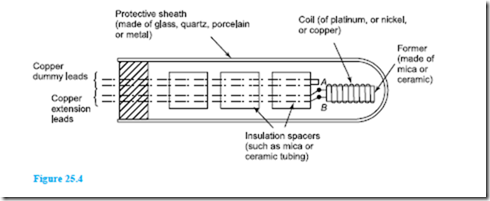
Resistance thermometers are made in a variety of sizes, shapes and forms depending on the application for which they are designed. A typical resistance thermometer is shown diagrammatically in Figure 25.4. The most common metal used for the coil in such thermometers is platinum even though its sensitivity is not as high as other metals such as copper and nickel. However, platinum is a very stable metal and provides reproducible results in a resistance thermometer. A platinum resistance thermometer is often used as a calibrating device. Since platinum is expensive, connecting leads of another metal, usually copper, are used with the thermometer to connect it to a measuring circuit.
The platinum and the connecting leads are shown joined at A and B in Figure 25.4, although sometimes this junction may be made outside of the sheath. How- ever, these leads often come into close contact with the heat source which can introduce errors into the measurements. These may be eliminated by including a pair of identical leads, called dummy leads, which experience the same temperature change as the extension leads.
Principle of operation
With most metals a rise in temperature causes an increase in electrical resistance, and since resistance can be measured accurately this property can be used to measure temperature. If the resistance of a length of wire at 0°C is R0, and its resistance at θ°C is Rθ, then Rθ = R0 (1 + αθ), where a is the temperature coefficient of resistance of the material.
Values of R0 and α may be determined experimentally or obtained from existing data. Thus, if Rθ can be measured, temperature θ can be calculated. This is the principle of operation of a resistance thermometer. Although a sensitive ohmmeter can be used to measure Rθ, for more accurate determinations a Wheat- stone bridge circuit is used as shown in Figure 25.5. This circuit compares an unknown resistance Rθ with others of known values, R1 and R2 being fixed values and R3 being variable. Galvanometer G is a sensitive centre-zero microammeter. R3 is varied until zero deflection is obtained on the galvanometer, i.e. no current flows through G and the bridge is said to be ‘balanced’.
A resistance thermometer may be connected between points A and B in Figure 25.5 and its resistance Rθ at any temperature θ accurately measured. Dummy leads included in arm BC help to eliminate errors caused by the extension leads which are normally necessary in such a thermometer.
Limitations
Resistance thermometers using a nickel coil are used mainly in the range –100°C to 300°C, whereas platinum resistance thermometers are capable of measuring with greater accuracy temperatures in the range –200°C to about 800°C. This upper range may be extended to about 1500°C if high melting point materials are used for the sheath and coil construction.
Advantages and disadvantages of a platinum coil Platinum is commonly used in resistance thermometers since it is chemically inert, i.e. un-reactive, resists corrosion and oxidation and has a high melting point of 1769°C. A disadvantage of platinum is its slow response to temperature variation.
Applications
Platinum resistance thermometers may be used as calibrating devices or in applications such as heat- treating and annealing processes and can be adapted easily for use with automatic recording or control systems. Resistance thermometers tend to be fragile and easily damaged especially when subjected to excessive vibration or shock.
Problem 2. A platinum resistance thermometer has a resistance of 25 W at 0°C. When measuring the temperature of an annealing process a resistance value of 60 W is recorded. To what temperature does this correspond? Take the temperature coefficient of resistance of platinum as 0.0038/°C
Exercise 138 Further problem on the resistance thermometer
1. A platinum resistance thermometer has a resistance of 100 W at 0°C. When measuring the temperature of a heat process a resistance value of 177 W is measured using a Wheatstone bridge. Given that the temperature coefficient of resistance of platinum is 0.0038/°C, determine the temperature of the heat process, correct to the nearest degree.
[203°C]
Related posts:
Incoming search terms:
- liquid in glass thermometer
- liquid in glass thermometer advantages and disadvantages
- working principle of liquid in glass thermometer
- on what thermometric property does the working of a thermistor depend
- liquid-in-glass thermometer
- physical properties of liquid in glass thermometer
- advantages and disadvantages of liquid in glass thermometer
- diagram of resistance thermometer
- liquid in gas thermometer
- measuring liquid temperature with thermociuple
- working of liquid in glass thermometer
- liquid in glass thermometer how it works
- liquid in glass thermometer diagram and the label
- mercury in glass thermometer diagram
- thermo electric thermometer
- clinical thermometer diagram
- errors in mercury thermometer
- principle of operation of liquid in glass thermometer
- diagram of a resistance thermometer
- the principle of operation of a liguid thermometer
- Differentiate between gas themometer and resistance thermometer
- Thermoelectric thermometer
- Explain the measurement of temperature using liquid in glass thermometer
- labelled diagram of liquid in glass thermometer
- diagram of resistant thermometer
- operation of liquid in gas thermometer
- diagram of mercury in glass thermometer
- diagram of glass thermometer
- temperature measurement using gas thermometer
- liquid in glass and thermocouple
- resistance thermometer diagram
- diagram of a thermoelectric thermometer in colour
- draw and label a thermometer
- UNIJUNCTION THERMOMETER
- liquid in glass thermometer diagram
- platinum resistance thermometer diagram
- well labelled diagram of a clinical thermometer
- a well labeled diagram of resistance thermometer
- A well labelled diagram of a clinical thermometer
- glass thermometer diagram
- advantages and disadvantages of liquid filled thermometer
- draw and label clinical thermometer
- Draw a diagram of:Thermo electric thermometer and Resistance thermometer
- differentiate between liquid in glass thermometer and optical pyrometer
- a well labelled diagram of thermometer
- drawing of liquid in a glass thermometer
- explain themometer
- draw and label mercury in glass thermometer
- featurs of liquid in glass thermometer
- filled system thermometer merit and demerits