REFRIGERANT PROPERTIES
Refrigerants can be characterized by a number of properties. These properties are pressure, temperature, volume, density, enthalpy, flammability, ability to mix with oil, moisture reaction, odor, toxicity, leakage tendency, and leakage detection.
Freon refrigerants R-11, R-12, and R-22, plus ammonia and water, will be discussed in terms of the above-mentioned categories. Freon R-11, R-12, and R-22 are common Freon refrigerants. The number as- signed to ammonia is R-717, while water has the number R-718.
Pressure
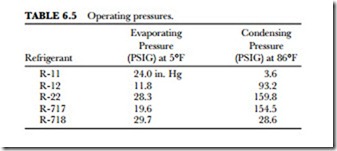
The pressure of a refrigeration system is important. It determines how sturdy the equipment must be to hold the refrigerant. The refrigerant must be compressed and sent to various parts of the system under pressure. The main concern is keeping the pressure as low as possible. The ideal low-side, or evaporating, pressure should be as near atmospheric pressure (14.7 pounds per square inch) as possible. This keeps the price of the equipment down. It also puts positive pressure on the system at all points. With a small pressure it is possible to prevent air and moisture from entering the system. In the case of a vacuum or a low pressure, it is possible for a leak to suck in air and moisture. Note the five refrigerants and their pressures in Table 6.5.
Freon R-11 is used in very large systems because it requires more refrigerant than others—even though it has the best pressure characteristics of the group. Several factors must be considered before a suitable refrigerant is found. There is no ideal refrigerant for all applications.
Temperature
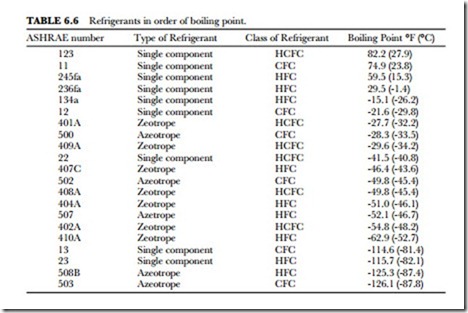
Temperature is important in selecting a refrigerant for a particular job. The boiling temperature is that point at which a liquid is vaporized upon the addition of heat. This, of course, depends upon the refrigerant and the absolute pressure at the surface of the liquid and vapor. Note that in Table 6.6, R-22 has the lowest boiling temperature. Water (R-718) has the highest boiling temperature. Atmospheric pressure is pounds per square inch.
Once again, there is no ideal atmospheric boiling temperature for a refrigerant. However, temperature–pressure relationships are important in choosing a refrigerant for a particular job.
Volume
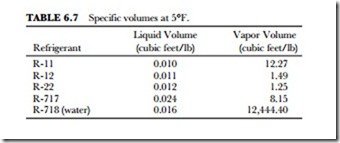
Specific volume is defined as the definite weight of a material. Usually expressed in terms of cubic feet per pound, the volume is the reciprocal of the density. The specific volume of a refrigerant is the number of cu- bic feet of gas that is formed when one pound of the refrigerant is vaporized. This is an important factor to be considered when choosing the size of refrigeration-system components. Compare the specific volumes (at 5 degrees F) of the five refrigerants we have chosen. Freon R-12 and R-22 (the most often used refrigerants) have the lowest specific volumes as vapors. (Refer to Table 6.7.)
Density
Density is defined as the mass or weight per unit of volume. In the case of a refrigerant, it is given in pounds per cubic foot (pounds/cubic foot). Note in Table 6.8 that the density of R-11 is the greatest. The density of R-717 (ammonia) is the least.
Enthalpy
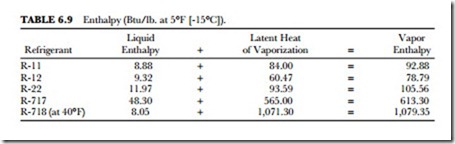
Enthalpy is the total heat in a refrigerant. The sensible heat plus the latent heat makes up the total heat. La- tent heat is the amount of heat required to change the refrigerant from a liquid to a gas. The latent heat of vaporization is a measure of the heat per pound that the refrigerant can absorb from an area to be cooled. It is therefore a measure of the cooling potential of the refrigerant circulated through a refrigeration system. (SeeTable 6.9.) Latent heat is expressed in Btu per pound.
Flammability
Of the five refrigerants mentioned, the only one that is flammable is ammonia. None of the Freon com- pounds are flammable or explosive. However, mixtures with flammable liquids or gases may be flammable and should be handled with caution. Partially halogenated compounds may also be flammable and must be individually examined. If the refrigerant is used around fire, its flammability should be carefully considered. Some city codes specify which refrigerants cannot be used within city limits.
Capability of Mixing with Oil
Some refrigerants mix well with oil. Others, such as ammonia and water, do not. The ability to mix with oil has advantages and disadvantages. If the refrigerant mixes easily, parts of the system can be lubricated eas-
ily by the refrigerant-oil mixture. The refrigerant will bring the oil back to the compressor and moving parts for lubrication.
There is a disadvantage to the mixing of refrigerant and oil. If it is easily mixed, the refrigerant can mix with the oil during the off cycle and then carry off the oil once the unit begins to operate again. This means that the oil needed for lubrication is drawn off with the refrigerant. This can cause damage to the compressor and moving parts. With this condition, there is foaming in the compressor crankcase and loss of lubrication. In some cases the compressor is burned out. Procedures for cleaning a burned-out motor will be given later.
Moisture and Refrigerants
Moisture should be kept out of refrigeration systems, since it can corrode parts of the system. Whenever low temperatures are produced, the water or moisture can freeze. If freezing of the metering device occurs, then refrigerant flow is restricted or cut off. The system will have a low efficiency or none at all. The degree of ef- ficiency will depend upon the amount of icing or the part affected by the frozen moisture.
All refrigerants will absorb water to some degree. Those that absorb very little water permit free water to collect and freeze at low-temperature points. Those that absorb a high amount of moisture will form corrosive acids and corrode the system. Some systems will allow water to be absorbed and frozen. This causes corrosion.
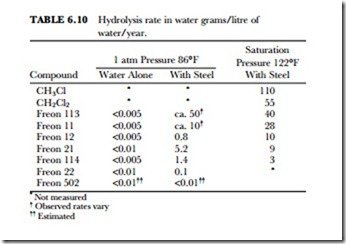
Hydrolysis is the reaction of a material, such as Freon 12 or methyl chloride, with water. Acid materials are formed. The hydrolysis rate for the Freon compounds as a group is low compared with other halogenated compounds. Within the Freon group, however, there is considerable variation. Temperature, pressure, and the presence of other materials also greatly affect the rate. Typical hydrolysis rates for the Freon compounds and other halogenated compounds are given in Table 6.10.
With water alone at atmospheric pressure, the rate is too low to be determined by the analytical method used. When catalyzed by the presence of steel, the hydrolysis rates are detectable but still quite low. At saturation pressures and a higher temperature, the rates are further increased.
Under neutral or acidic conditions, the presence of hydrogen in the molecule has little effect on the hydrolytic stability. However, under alkaline conditions compounds containing hydrogen, such as Freon 22 and Freon 21, tend to be hydrolyzed more rapidly.
Odor
The five refrigerants are characterized by odor or the absence of odor. Freon R-11, R-12, and R-22 have a slight odor. Ammonia (R-717) has a very acrid odor and can be detected even in small amounts. Water (R-718), of course, has no odor.
A slight odor is needed in a refrigerant so that leakage can be detected. A strong odor may make it im- possible to service equipment. Special gas masks may be needed. Some refrigerated materials may be ru- ined if the odor is too strong. About the only time that an odor is preferred in a refrigerant is when a toxic material is used. A refrigerant that may be very inflammable should also have an odor so that leakage can be detected easily to prevent a fire or explosion.
Toxicity
Toxicity is the characteristic of a material that makes it intoxicating or poisonous. Some refrigerants can be very toxic to humans; others may not be toxic at all. The halogen refrigerants (R11, R-12, and R-22) are harmless in their normal condition or state. However, they form a highly toxic gas when an open flame is used around them.
Water, of course, is not toxic. Ammonia can be toxic if present in sufficient quantities. Make sure the manufacturer’s recommended procedures for handling are followed when working with refrigerants.
Tendency to Leak
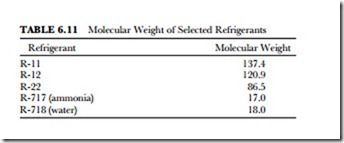
The size of the molecule makes a difference in the tendency of a refrigerant to leak. The greater the molecular weight, the larger the hole must be for the refrigerant to escape. A check of the molecular weight of a refrigerant will indicate the problem it may present to a sealed refrigeration system. Table 6.11 shows that R-11 has the least tendency to leak, whereas ammonia is more likely to leak.
Related posts:
Incoming search terms:
- refrigerant properties
- properties of hvac refrigerant
- the tendency of leak is more if the molecule weight of refrigerant
- the tendency of leak is more if the molecular weight of refrigerant
- tendency of leak is more if the molecular weight of refrigerant ?
- tendency of leak is more if the molecular weight of refrigerant
- definition of leak tendency in refrigeration
- lowest sp volume refrigrent
- freon refrigerant r-11
- freon refrigerant gas property
- the tendency of of leak is more if the molecular weight of refrigerant