Mass and Mole Balances
Due to chemical reactions, the mole numbers of all components involved in the reaction change. The reaction rate λ is defined as the number of net reactions (counted in moles) per unit time and volume. The reaction rate can be positive or negative, depending whether forward or backward reactions prevail. For each mole of reactions there are γα moles of component α produced or consumed. The rate of change of mole number for component α reads
where the summation has to be taken over all substances involved in the reaction, that is products and reactants. Thus, the mass of the products is equal to the mass of the reactants,
A third equation, for O2 and H2, is not shown; this equation is a linear combination of those above, and does not give additional information.
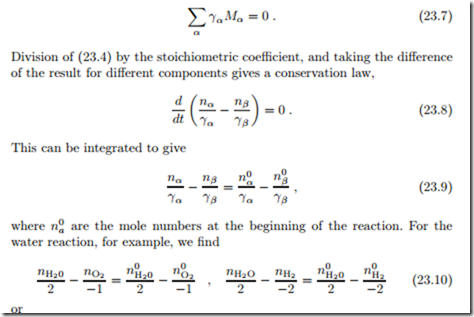
Alternatively, one can balance the mole numbers of elements, since these must be conserved (nuclear reactions excluded). The only elements in the water reaction are oxygen and hydrogen, and the conservation of elements gives rise to the equations
Obviously, this is equivalent to the previous equations. Balancing of elements must be used when multiple reactions occur.