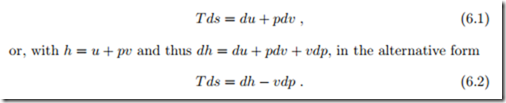State Properties and Their Relations
The thermodynamic laws contain many state properties, e.g. [SI units in brackets]
 However, only few properties (T, p, m, V, V) can be measured directly, while many of the quantities that appear in the thermodynamic laws (u, h, s,.. .) cannot be measured directly.
However, only few properties (T, p, m, V, V) can be measured directly, while many of the quantities that appear in the thermodynamic laws (u, h, s,.. .) cannot be measured directly.
Experience shows that state properties are not independent, but are related through property relations, which depend on the substance. By means of property relations, thermodynamic quantities (u, h, s,.. .) can be determined indirectly, through measurement of (T, p, m, V, V).
Measurements show that for simple substances it is sufficient to know two properties to find all others. This implies property relations of the form
and so on. The thermal and caloric equations of state, p (T, v) and u (T, v), must be determined in careful measurements, where the measurement of the latter relies on the first law. In most cases, the equations of state are not given as explicit equations, but in form of tables. The best known exception is the ideal gas law, p = RT /v.
Entropy must be determined from the thermal and caloric equations of state through integration of the Gibbs equation, which gives a differential relation between properties, and holds for all simple substances in the form
Property relations can be formulated between any set of three properties. For instance: Considering the entropy as function of temperature and pressure, s (T, p), together with the thermal equation of state, p (T, v), both can be combined to s (T, p (T, v)) = s (T, v), that is entropy as function of temperature and volume. Inversion of the caloric equation of state u (T, v) for temperature yields temperature as a function of energy and volume, T (u, v). Considering the latter in the entropy expression s (T, v) yields entropy as function of energy and volume, s (u, v). Solving this relation for energy, yields energy as a function of entropy and volume, u (s, v). And so on. These are just some examples of variable changes in property relations. A detailed analysis of property relations, where variable changes are used to identify deeper relations between properties can be found in Chapter 16, where it will be seen that the Gibbs equation substantially reduces the measurements necessary to produce thermodynamic tables.