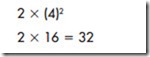To understand electricity, one should start with the study of atoms. The atom is the basic building block of the universe. All matter is composed of atoms. An atom is the smallest part of any element. Atoms are composed of three principal parts, the electron, the proton, and the neutron. Electrons exhibit a nega- tive charge, protons exhibit a positive charge, and neutrons have no charge. Some theories suggest that neutrons are composed of both a proton and an electron. The positive charge of the proton and the negative charge of the electron cancel each other.
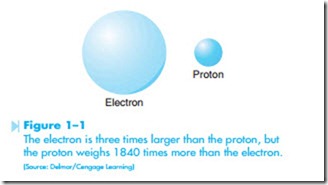
Protons and neutrons are extremely massive particles as compared to the electron. It is believed that the electron is approximately three times larger than a proton, but the proton weighs approximately 1840 times more than an electron, as shown in Figure 1–1. It is like comparing a piece of lead shot to a soap bubble.
THE LAW OF CHARGES
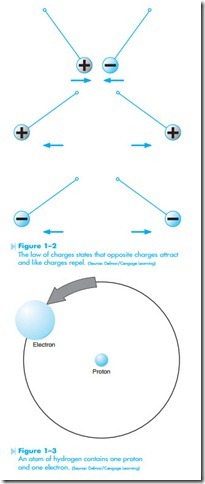
One of the basic laws of physics concerning atoms is the law of charges. This law states that opposite charges attract each other and like charges repel each other. If charged particles were suspended from a string, a positively charged particle and negatively charged particle would attract each other, but two positively charged particles or two negatively charged particles would repel each other, as shown in Figure 1–2. Since electrons are negatively charged particles and protons are positively charged particles, they are attracted to each other.
STRUCTURE OF THE ATOM
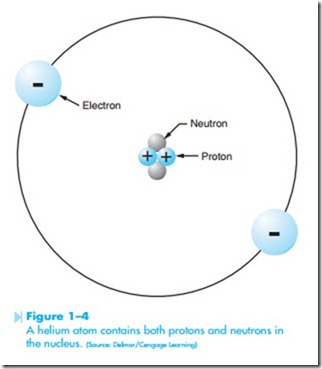
The nucleus or center of the atom is composed of protons and neutrons. Electrons orbit the exterior of the atom in specific orbits or shells. The smallest of all atoms is hydrogen. Hydrogen does not contain a neutron in its nucleus. The hydrogen atom contains one proton and one electron, as shown in Figure 1–3. The smallest atom that contains both protons and neutrons in the nucleus is helium, shown in Figure 1–4. Helium contains two protons, two neutrons, and two electrons.
In 1808 a scientist named John Dalton proposed that all matter was composed of atoms. Although the assumptions that Dalton used to prove his theory were later found to be factually incorrect, the idea that all matter is composed of atoms was adopted by most of the scientific world. Then in 1897 J. J. Thompson discovered the electron. Thompson determined that electrons have a negative charge and that they have very little mass compared to the atom. He proposed that atoms have a large,
positively charged, massive body with negatively charged electrons scattered throughout it. Thompson also proposed that the negative charge of the electrons exactly balanced the positive charge of the large mass, causing the atom to have a net charge of zero. Thompson’s model of the atom proposed that electrons existed in a random manner within the atom, much like firing BBs from a BB gun into a slab of cheese. This was referred to as the plum pudding model of the atom.
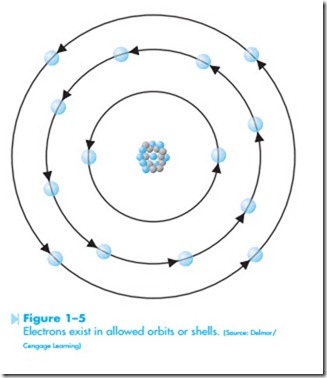
In 1913 a Danish scientist named Niels Bohr presented the most accepted theory concerning the structure of an atom. In the Bohr model, electrons exist in specific or “allowed” orbits around the nucleus, similar to the way that planets orbit the sun, as shown in Figure 1–5. The orbit in which the electron exists is determined by the electron’s mass times its speed times the radius of the orbit. These factors must equal the positive force of the nucleus. In theory there can be an infinite number of allowed orbits.
When an electron receives enough energy from some other source it “quantum jumps” into a higher allowed orbit. Electrons, however, tend to return to a lower allowed orbit. When this occurs, the electron emits the excess energy as a single photon of electromagnetic energy.
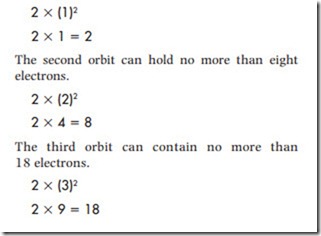
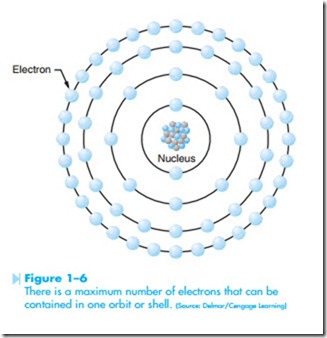
Atoms have a set number of electrons that can be contained in one orbit or shell, as shown in Figure 1–6. The number of electrons that can be contained in any one shell is determined by the formula (2N 2 ). The letter N represents the number of the orbit or shell. For example, the first orbit can hold no more than two electrons.
The fourth and fifth orbits cannot hold more than 32 electrons. Thirty-two is the maximum number of electrons that can be contained in any orbit.
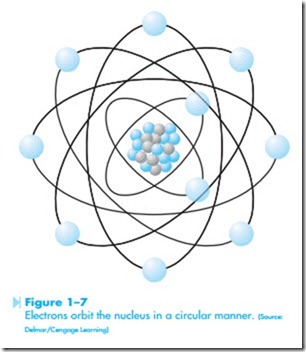
Although atoms are often drawn flat, as illustrated in Figure 1–6, the electrons orbit around the nucleus in a circular fashion, as shown in Figure 1–7. The electrons travel at such a high rate of speed that they form a shell around the nucleus. It would be similar to a golf ball being surrounded by a tennis ball that is surrounded by a basketball. For this reason, electron orbits are often referred to as shells.
VALENCE ELECTRONS
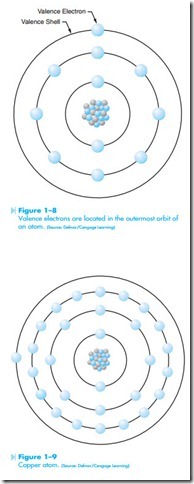
The outermost shell of an atom is known as the valence shell. Any electrons located in the outer shell of an atom are known as valence electrons, as shown in Figure 1–8. The valence shell of an atom cannot hold more than eight electrons. It is the valence electrons that are of primary concern in the study of electricity, because it is these electrons that explain much of electrical theory. A conductor, for instance, is made from a material that contains one or two valence electrons. Atoms with one or two valence electrons are unstable and will give up these electrons easily. Conductors are materials that permit electrons to flow through them easily. Silver, copper, and gold all contain one valence electron and are excellent conductors of electricity. Silver is the best natural conductor of electricity, followed by copper, gold, and aluminum. An atom of copper is shown in Figure 1–9. Although it is known that atoms containing few valence electrons are the best conductors, it is not known why some of these materials are better conductors than others. Copper, gold, platinum, and silver all contain only one valence electron. Silver, however will con- duct electricity more readily than any of the others. Aluminum, which contains three valence electrons, is a better conductor of electricity than platinum, which contains only one valence electron.
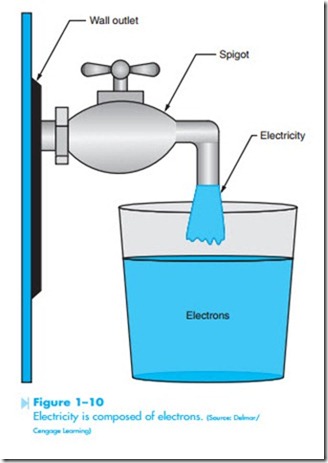
Electricity is composed of electrons. If it were possible to connect a spigot to a wall outlet and catch electricity in a glass, the glass would be full of electrons, as shown in Figure 1–10. Electric current is the flow of electrons, which means that
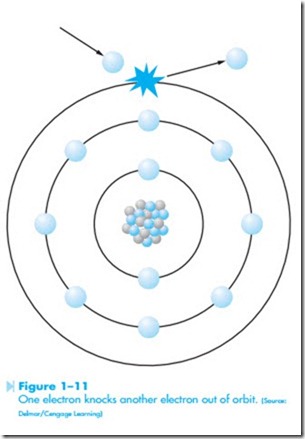
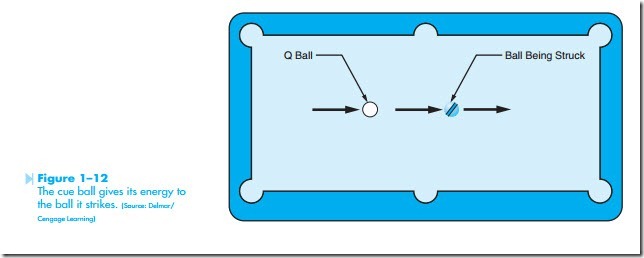
the electrons must be moving to produce current. It is the movement of the electrons that produces electrical energy. There are several theories con- cerning how electrons are made to flow through a conductor. One theory is generally referred to as the bump theory. It states that current flow is produced when an electron from one atom knocks electrons of another atom out of orbit, as shown in Figure 1–11. The striking electron gives its energy to the electron being struck. The striking electron settles into orbit around the nucleus, and the electron that was struck moves off to strike another electron. This action can often be seen in the game of pool. The moving cue ball gives it energy to the ball being struck, as shown in Figure 1–12. The stationary ball then moves off with most of the energy of the cue ball, and the cue ball stops moving. Although most of the energy was transferred to the stationary ball, some energy was dissipated as heat caused by the impact of the cue ball striking the stationary ball.
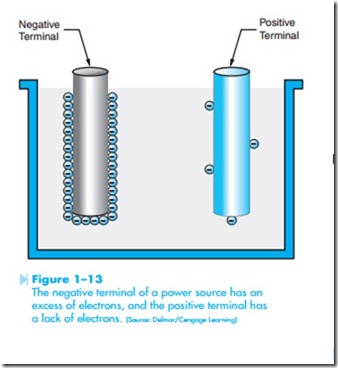
Other theories deal with the fact that all electric power sources produce a positive and negative
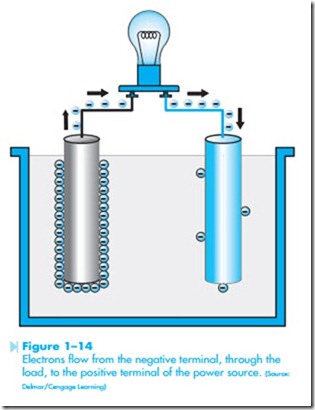
terminal. The negative terminal is created by causing an excess of electrons to form at that terminal, and the positive terminal is created by removing a large number of electrons from that terminal, as shown in Figure 1–13. Different methods can be employed to produce the excess of electrons at one terminal and deficiency of electrons at the other, but when a circuit is completed between the two terminals, negative electrons are repelled away from the negative terminal and attracted to the positive terminal, as shown in Figure 1–14. The greater the difference in the number of electrons between the negative and positive terminals, the greater the force of repulsion and attraction.
Most of the world’s electric power is produced by rotating machines called alternators. Alternators work on the principle of cutting magnetic lines of flux with a conductor. One of the basic principles of electricity is that anytime a conductors cuts magnetic lines of flux, a voltage is inducted into the conductor. Another common source of electricity is batteries. Batteries produce electricity by chemical action. Other methods include solar cells, thermocouples, and piezoelectric devices. Solar cells produce electricity when they receive photons of light that cause electrons to move. Thermocouples produce electricity by heating the junction of two different types of metal, and the piezoelectric effect produces current flow from pressure.
INSULATORS
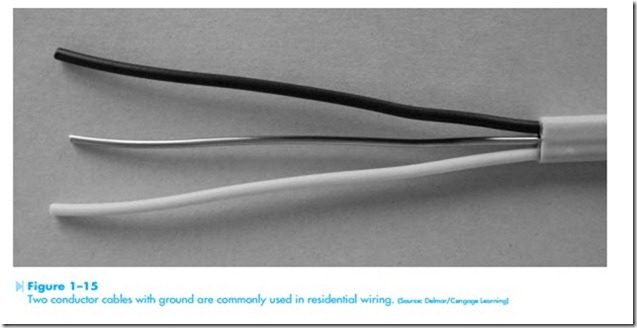
Insulators are materials that do not permit electrons to flow through them easily. The atoms of an insulator generally contain seven or eight valence electrons. When an atom contains seven or eight valence electrons, they are tightly held by the atom and not easily given up. Insulator materials are generally made from compounds instead of a pure element. The molecules of the compounds are extremely stable and do not break their bond easily. Some examples of insulators are wood, ceramic, mica, rubber, and thermoplastic. Insulator materials are generally rated by voltage. The voltage rating indicates the amount of volt- age they can withstand without breaking down. A very common electric cable used in residential wiring is the nonmetallic two-conductor cable with ground, shown in Figure 1–15. This cable is generally referred to as NM or Romex. The insula- tion around the conductors has a voltage rating of 600 volts.