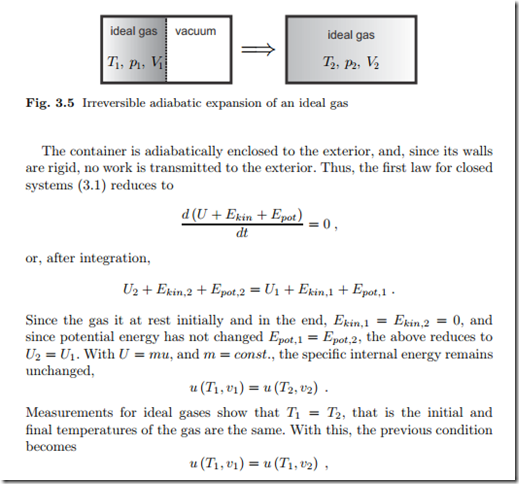
Example: Uncontrolled Expansion of a Gas
Our next example concerns the uncontrolled expansion of an ideal gas. For this, we consider an ideal gas in a container which is divided by a membrane, see Fig. 3.5. Initially the gas is contained in one part of the container at {T1, p1, V1}, while the other part is evacuated. The membrane is destroyed, and the gas expands into the container. The fast motion of the gas is slowed down by internal friction, and in the final homogeneous equilibrium state {T2, p2, V2} the gas is at rest and distributed over the total volume of the container. Note that we have no control over the flow after the membrane is destroyed: this is an irreversible process.
which can only hold if the internal energy of the ideal gas does not depend on volume. This experiment verifies that the internal energy of the ideal gas is independent of volume, and depends only on temperature, u = u (T ).