LARGE-SCALE BATTERIES
Batteries are the most widely known means of storing electrical energy. Invented during the 19th century, they are now used for a whole range of portable applications from starter motor power in vehicles to providing an electrical source for mobile phones, tablet computers, and tiny electronic devices such as hearing aids. More recently, batteries have also been used in a range of mostly small renewable energy applications, and in addition some large cells have been used for grid storage and stability uses.
All batteries are electrochemical devices that convert the energy that is released during a variety of chemical reactions into electrical energy. This energy would normally be released as heat if the reaction was permitted to proceed conventionally by mixing the reactants. In an electrochemical cell (i.e., a battery) the reaction is controlled in such a way that most of this heat can be converted into electricity.
There are two distinct battery types in common use: primary cell and secondary cell. A primary cell (or battery) can only be used once. After that it can- not be recharged. However, a secondary cell is capable of being recharged, reversing the internal chemical reaction and regenerating the reactants that pro- vided the power in the first place. It is these secondary cells that are of use in the power and utility industries.
Secondary cells can be further divided into two types: standard secondary cells and flow batteries. Standard cells are the type found in portable computers or vehicles. They are completely self-contained, and have no mechanical parts. Charging and discharging is carried out via the cell terminals and all of the reac- tants required are contained within the battery package. Secondary cells can be found in two types: shallow discharge cells, such as those used for vehicle starter power, which are never fully discharged, and deep discharge cells that can be completely exhausted without damage.
A flow battery is different because the actual cell within which the chemical reactants react and generate electricity does not carry the reactants themselves. Instead, these are stored in external reservoirs and pumped through the cell as required. This type of battery is more complex than a conventional secondary cell, but it has the advantage that battery capacity is limited by reservoir size and this can easily be increased for relatively little cost. On the other hand, flow batteries tend only to be economical in large sizes because of the additional cost associated with their construction.
Battery Principle
A battery is a device that can exploit a chemical reaction to produce electricity. The reactions upon which a cell is based will define the particular cell type. In all cases the reaction will occur spontaneously if the reactants are mixed, generating heat in the process. However, in the battery the reactants are separated and only allowed to react in a particular way.
The chemical reaction used in every battery can notionally be divided into two half-reactions and the battery will contain two electrodes, called the anode and cathode; each electrode is associated with one of these half-reactions. The half-reactions involve the creation of charged ions and the capture or release of electrons. Under normal circumstances where the reactants are intimately
mixed, these processes occur simultaneously at the same location. However, in a battery the two electrodes are separated by an electrolyte that will allow charged ions to pass from one electrode to the other but will not allow electrons to pass. These can only cross from one electrode to the second to complete the reaction through an external circuit. This is the electrical current that can be used to drive electrical and electronic equipment. It is this separation of processes by the use of a selective filter (in this case, the electrolyte) that allows the cell to generate power.
As mentioned, batteries are generally divided into primary cells and secondary cells. Primary cells contain reactants that will only react once to produce power. After that the cell is spent. A secondary cell can be recharged by apply- ing a reverse polarity to the cell, reversing the chemical cell reaction and regenerating the original cell reactants. It is this type of cell that is of use for energy storage.
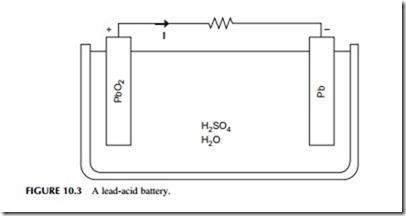
There are a number of battery types that can be used for grid and utility applications. The most widely used secondary cell is the lead-acid battery, similar to the type commonly used in vehicles (Figure 10.3). Lead-acid batteries account for roughly half of all secondary cell sales, globally, and are widely available. Another type, nickel-cadmium (NiCad) batteries, are less common. NiCad batteries were used for portable computers until they were superseded by alternatives that were better suited to the duty cycle of such devices. They have also been used for automotive applications, particularly under low- temperature conditions when they behave more reliably than lead-acid cells. Other cell types developed specifically for portable electronic devices include nickel-metal hydride cells and lithium-ion cells. Both are potentially useful for utility storage. A high-temperature battery, the sodium-sulfur battery, has also been popular for utility applications.
In addition to these conventional secondary cells, a variety of devices known as flow cells or flow batteries have also been tested for large-scale energy
storage applications. These include zinc-bromide, vanadium-redox, and polysufide-bromide flow cells. None has so far found widespread commercial application but new types are being developed. They are considered attractive because they have much longer lifetimes than conventional secondary batteries.
Traditional electrochemical storage systems boast a best-case cycle conver- sion efficiency (electricity to cell storage and back into electricity) of 90%, but a more typical figure would be 70%. Most batteries also suffer from leakage of power over time. Left for too long, the cell discharges itself. This means that battery systems can only be used for relatively short-term storage. Flow cells do not suffer from this problem because the reactants are not stored together and this helps reduce long-term energy losses.
An additional problem with traditional secondary batteries is their tendency
to age. After a certain number of cycles, the cell stops holding its charge effectively, or the amount of charge it can hold declines. Much development work has been aimed at extending the lifetime of electrochemical cells but this remains a problem. Again flow cells, because of their design, can avoid this problem.
Against this, batteries are able to supply their output extremely quickly— under 5 msec for a conventional battery and less than 100 msec for a flow battery. Some are also capable of very high-power outputs and discharge rates.
Lead-Acid Batteries
Lead-acid batteries were among the first secondary cells to be developed and were used for load leveling in very early power distribution systems. The cell is based on a reaction between lead-oxide and sulfuric acid. Efficiencies of lead- acid batteries vary depending on factors such as the temperature and duty cycle but are typically between 75% and 85% for DC–DC cycling. However, cells discharge themselves over time so they cannot be used for very long-term power storage. If cycled carefully, cells for utility applications can have lifetimes of 15–30 years.
The cells have a water-based liquid electrolyte and operate at ambient temperature. Both high and low temperatures can reduce their performance. They are also relatively heavy and have a poor energy density, although neither of these factors are a handicap for stationary applications. In addition, they are cheap and easily recycled.
Several very large energy storage facilities based on lead-acid batteries have been built. These include and 8.5 MW unit constructed in West Berlin in 1986 while the city was still divided into East and West, and a 20 MW unit built in Puerto Rico in 1994. While the former operated successfully for several years, cell degradation led to the latter closing after only five years. Lead-acid cells have been very popular for renewable applications such as small wind or solar installations where they are used to store intermittently generated power to make it continuously available.
Nickel-Cadmium Batteries
The nickel-cadmium battery is one of a family of nickel batteries that includes nickel–metal hydride, nickel-iron, and nickel-zinc batteries. There is also a nickel-hydrogen battery in which one cell reactant is gaseous hydrogen. All have a nickel electrode coated with a reactive and spongy nickel hydroxide while the cell electrolyte is almost always potassium hydroxide. Cell reactions vary depending on the second component.
The only nickel-based cell that has been exploited for utility applications is the nickel-cadmium cell. Nickel-cadmium batteries have higher energy densi- ties and are lighter than lead-acid batteries. They also operate better at low temperatures. However, they tend to be more expensive. This type of battery was used widely in portable computers and phones but has now been superseded by lithium-ion batteries.
Efficiencies of nickel-cadmium cells are typically around 70% although some have claimed up to 85%. Lifetimes tend to be rated at around 10–15 years though some have lasted longer. These cells discharge themselves more rapidly than lead-acid cells and can lose 5% of their charge in a month. There can also be a problem with disposal since cadmium is highly toxic.
The largest nickel-cadmium battery ever built is a 40 MW unit in Alaska that was completed in 2003. It occupies a building the size of a football field and comprises 13,760 individual cells.
Lithium Batteries
Lithium batteries, including both lithium-hydride and lithium-ion batteries, have become popular for consumer electronic devices because of their low weight, high energy density, and relatively long lifetimes. Lithium is extremely reactive and can burst into flames if exposed to water, but modern lithium cells use lithium bound chemically so that it cannot react easily. As with nickel, there are a number of lithium cell variants but the most popular today is the lithium- ion cell. These are designed so that there is no free lithium present at any stage during the charging or discharging cycle.
The use of lithium batteries in grid and utility applications is beginning to grow with units being tested in a number of locations. One large installation, due to start operating in 2013, is a 2 MW lithium-ion facility for the Orkney Islands off the northwestern coast of Scotland. The future development of lithium batteries may benefit from interest by automotive manufacturers in their use in hybrid and electric vehicles.
Sodium-Sulfur Batteries
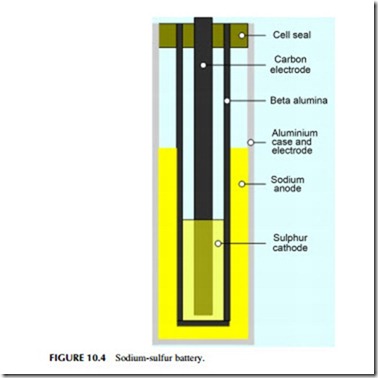
The sodium-sulfur battery is a high-temperature battery (Figure 10.4). It operates at 300 oC and utilizes a solid electrolyte, making it unique among the com- mon secondary cells. One electrode is molten sodium and the other molten
sulfur, and it is the reaction between these two that is the basis for the cell reaction. Although the reactants, and particularly sodium, can behave explosively, modern cells are generally reliable. However, a fire was reported in 2012 at a sodium-sulfur battery installation in Japan.
Early work on the sodium-sulfur battery took place at the Ford Motor Co. in the 1960s but modern sodium-sulfur technology was developed in Japan by the Tokyo Electric Power Co., in collaboration with NGK insulators, and it is these two companies that have commercialized the technology. Typical units have a rated power output of 50 kW and 400 kWh. Lifetime is claimed to be 15 years or 4500 cycles and the efficiency is around 85%. Sodium-sulfur batteries have one of the fastest response times, with a startup speed of 1 msec.
Flow Batteries
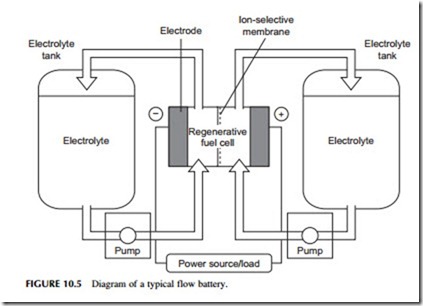
A flowing-electrolyte battery, or flow battery, is a cross between a conventional battery and a fuel cell. It has electrodes like a conventional battery, where the electrochemical reaction responsible for electricity generation or storage takes place, and an electrolyte. However, the chemical reactants responsible for the electro- chemical reaction and the product of that reaction are stored in tanks separate from
the cell and pumped to and from the electrodes as required, much like a fuel cell (Figure 10.5). The processes occurring here are usually somewhat different to the simple electrochemical process described earlier but the principle is similar.
A number of flow batteries have been tested including the zinc-bromide flow battery, the polysulfide-bromide battery, and the vanadium redox battery. A number of newer designs are also in the research stage. Response times for flow batteries are longer than for conventional secondary batteries but they should be able to supply full power within 100 msec. However, flow batteries have not been extensively tested commercially so their overall performance has yet to be established.
Costs
The costs of battery systems vary widely depending on type and size. Large batteries are generally cheaper than similar small installations. Battery costs are normally based on the cost per kilowatt-hour rather than the cost per kilowatt. The cheapest of the conventional cells is the sodium-sulfur battery with a cost in the range of $250–900/kWh. Lead-acid battery costs are in the range of $400– 2300/kWh and nickel-cadmium of $1800–2400/kW. Flow batteries are potentially cheaper with costs in the range of $150–800/kWh depending on the type.