Moisture
The presence of moisture in gases or solids often needs to be sensed or measured. A high moisture content in a gas (high humidity) will cause condensation when the gas is cooled, and this can have the effect of depositing liquid in pipes, causing blockages. Conversely, a gas whose humidity is very low can absorb moisture from joints in a pipe, causing the joints to dry up and leak. The moisture content in solids may be desirable, as in the preservation of antique furniture or the working of textiles, or undesirable when mould starts to appear on brickwork. All of this assumes that the moisture is water, but in some specialized applications the presence of other liquids may need to be sensed, and such applications require equipment that is much more specialized.
The presence of moisture (water) in a gas is termed humidity, and the absolute humidity of a gas (usually air) is the mass of water per unit mass of gas. This absolute humidity is the quantity that needs to be known if the amount of water that can be condensed from a gas has to be determined. The amount of water that can be contained in a gas is limited, and the maximum humidity attainable is called the saturation humidity. This figure depends heavily on the temperature, being very low at low gas tem-peratures (around 0oC) and very high at temperatures approaching the boiling point of water (l00oC).
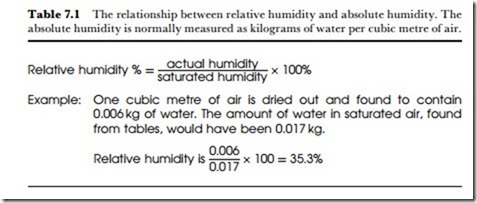
For many purposes, the relative humidity is a more important value than the absolute humidity. The relative humidity at any temperature is the absolute humidity divided by the value of saturated humidity at that tem- perature, usually expressed as a percentage (Table 7.l). A relative humidity value of 50% at 20oC, for example, means that the air contains
half of the quantity of water that would be needed to saturate it at this tem- perature. Calculations of absolute humidity from values of relative humidity are never simple because tables of saturated humidity always quote vapour pressures, and Table 7.2 shows how these values can be used to obtain absolute figures.
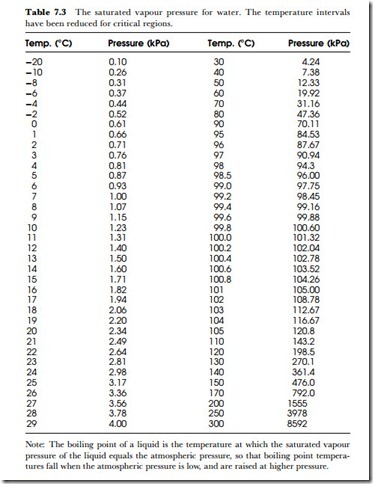
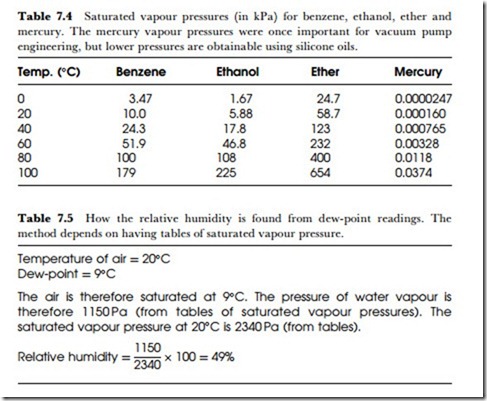
These calculations depend on using tables of saturated vapour pressure at different temperatures, and such tables have been in use for several centuries now, particularly for steam engineering. Table 7.3 includes the critical values around the boiling point, and the pressures for superheated steam up to 300oC. Tables of vapour pressure for other liquids are not so easy to come by. A few are listed in Table 7.4.
Another measure of relative humidity is the dew-point. When a surface is cooled in contact with a gas, a temperature will eventually be reached when the gas deposits water onto the surface by condensation. This surface temperature is called the dew-point temperature, and its significance is that it corresponds to the temperature at which the gas would be saturated. If the dew-point temperature is known, then the vapour- pressure of the water in the gas can be found from tables, and either the relative or the absolute humidity calculated, as illustrated in Table 7.5.
Electronic methods of measuring humidity are based either on dew-point or on the behaviour of moist materials. Of these, methods that use moist materials are by far the simplest to use, although they need to be calibrated at intervals if they are used for measurement as opposed to sensing purposes. The simplest method is a very old one, using the principle of the hair hygrometer. A human hair, washed in ether to remove all traces of oil or grease, is very strongly affected by humidity, and its length will shrink in dry conditions and expand in moist conditions.
A very satisfactory relative humidity sensor can therefore be constructed by using a slightly tensioned hair along with any of the standard methods for detecting length changes, such as the linear potentiometer, LVDT, capacitive (with oscillator) and so on. As usual, the use of a capacitive
gauge allows the reading to be made in terms of change of frequency and makes it easy to treat the information by digital methods. The amount of force on the hair must be very small, so that whatever method of sensing length changes is used must not place an excessive load on the hair. The Table 7.4 Saturated vapour pressures (in kPa) for benzene, ethanol, ether and mercury. The mercury vapour pressures were once important for vacuum pump engineering, but lower pressures are obtainable using silicone oils.
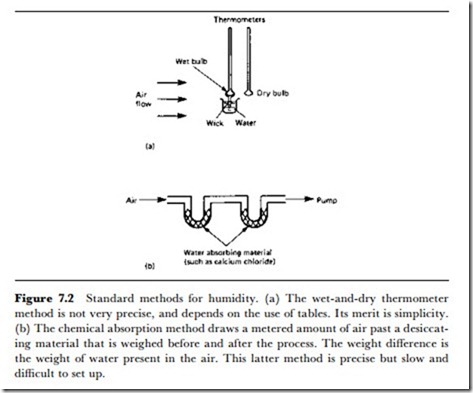
system can be calibrated either against another indicating hygrometer, or against a standard chemical humidity measurement (Figure 7.2).
Although the hair type of hygrometer is capable of surprisingly good results, the lithium chloride type is more directly suitable for electronic purposes. Lithium chloride has a very high resistance in its dry state, but the resistance drops considerably in the presence of water, and over a reasonable range the resistance reading can be used to measure relative humidity. The lithium chloride cell is made part of a measuring bridge, and the output is used to drive a meter movement, trigger a switch action at some critical value, or is converted to digital form for display.
Other effects can be used to determine the moisture content in absolute terms. The presence of moisture in air alters its value of permittivity, so that the capacitance between two fixed metal plates in air will change very slightly as the moisture content of the air changes. This, as usual, can be sensed in the form of a change of frequency of an oscillator. The readings have to be corrected for changes of temperature, since the dimensions of the plates will change as the temperature around them changes. Another effect that can be used is the heat conductivity of the air that will alter as the moisture content is altered.
The most useful effect, particularly for remote measurement, is
(a) The chemical absorption method draws a metered amount of air past a desiccating material that is weighed before and after the process. The weight difference is the weight of water present in the air. This latter method is precise but slow and difficult to set up.
microwave measurement. Microwaves at a frequency of 2.45 GHz are absorbed strongly by water (the principle of microwave cooking). If microwave signals at this frequency are beamed across air and returned from a fixed reflector, the amount of returned signal will depend very considerably on the water content in the intervening air. This method has the considerable advantage that it senses the average water content along a path in air, which can be quite large. The system needs to be calibrated, but once calibrated, the amplitude of the returning signal can be used as a measure of humidity (the greater the returned signal, the lower the humidity). Dew-point methods can provide fairly precise measurements of either absolute or relative humidity, but require the addition of a microprocessor system if direct readings are needed.
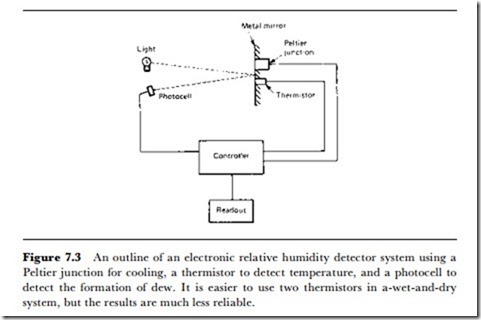
An outline of such a system is illustrated in Figure 7.3. This uses a Peltier junction as a cooling element, using the well-known principle that a pair of junctions between metals, which provides an EMF for an applied temperature difference will also operate in reverse, providing a temperature difference, when connected to a supply. When semiconductor junctions are used, the change in temperature when current is passed can be large enough to allow one junction to attain temperatures lower than 0oC while the other junction is held at room temperature. Since the effect is current-controlled, the rate of fall of temperature can be controlled electronically.
The Peltier junction is therefore used to lower the temperature, and a thermistor measures this temperature. A beam of light is reflected from the mirror-surface of the metal attached to the junction, and a photocell senses the reflected light. At the dew-point, the mist on the surface causes the amount of reflected light to drop sharply. The amplifier connected to the photocell operates the reading gate of the system, so that the output from the thermistor temperature gauge is converted to digital form and stored. The microprocessor system also stores the output from another thermistor gauge which senses room temperature. Its ROM contains a set of table values for the temperature readings and for relative or absolute humidity readings (the absolute humidity can be found only if the volume of the space is known). The method of obtaining relative humidity has been outlined in Table 7.5.
As an example of a room humidistat, the RS type 33l-ll8 uses a hair element which permits a setting range from 30-90% relative humidity (RH), with a switching differential of l.5% based on 45% RH. The calibration holds good up to an air temperature of 40oC and maximum air speed of l.5 m/s. The switch is rated at 480 V, l0 A AC or 250 V, 0.5 A DC. A more specialized unit for air-conditioned spaces uses a probe to measure both temperature and humidity, and the sensing principle for humidity is the capacitor type. The humidity range is l0-90% RH, with a tolerance of ±5%, and the unit is available in four versions, with low- voltage supplies of l2-30 V required. A more specialized unit for high- pressure gases uses a remote probe for both temperature and RH readings at pressures up to l0 MPa (l450 psi), with an RH range of 0-l00%.
MOISTURE IN SOLIDS
Moisture in solids can be sensed in terms either of the conductivity of the materials, changes in permittivity, or of the absorption of microwaves. For sensing the presence of moisture in materials of comparatively fixed composition (even where some variation occurs, as in masonry), a simple resistance reading between connectors set at a fixed distance is often all that is required. For measurement purposes, readings have to be calibrated, and calibration is a long and tedious procedure that requires samples with various moisture levels to be checked for resistance, then weighed, baked to remove moisture and weighed again. The difference in weights shows the moisture content which can be expressed as a percentage of the original weight of material.
A very common requirement is for moisture content of soil. This is very seldom required to be precise, which is just as well, because the relationship between the resistance of the soil and its moisture content is not a simple one. A simple resistance indication, however, is enough to tell whether a plant needs watering or not, which is the main reason for using moisture indicators. For civil engineering purposes, the use of a soil-resistance moisture meter is only a preliminary indicator, and a complete soil sample analysis would be needed before any decisions on the suitability of soil for foundations were made.