Competitor theories of heat energy
Our present thought regarding the nature of heat began to be put on a firm basis only from about the middle of the nineteenth century. Before then, discussion on the nature of heat had been going on among men of science for more than 250 years.
It is interesting to trace the path of investigations which led to the development of our modern viewpoint.
Robert Boyle explained this by saying that the blows of the hammer set the molecules of the nail into violent vibration, and therefore they become hot.
This view, of course, comes very close to the present-day impression of internal energy. But while the seventeenth-century scientists understood the meaning of motion and force, it did not occur to them to combine the two and think in terms of work or motion against force.
In brief, the ideas of work and energy as we understand them today had not yet made their appearance in the kingdom of mechanics and physics.
Heat came to be considered as a weightless fluid called caloric. This idea was more acceptable to men such as James Black, who found it easier to talk about heat in terms of a quantity of caloric rather than a vague quantity of motion.
And so the caloric theory gained ground and was destined to occupy a prominent place in the study of heat for the next 150 years.
Count Rumford and the revival of the motion theory of heat
In spite of the popularity of the caloric theory during the eighteenth century, there were some who did not find it altogether satisfactory. Its main critic was an American named Benjamin Thompson, who, for his services to the Elector of Bavaria, was later given the title of Count Rumford.
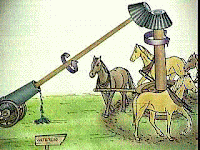 |
| Count Rumford’s cannon experiment |
During the time he was in charge of the arsenal at Munich, Rumford noticed that a great deal of heat was produced during the boring of brass cannon.
According to the caloric theory, this was caused by caloric being squeezed out of the metal by the action of the boring tool.
This loss of caloric could be explained by supposing that the metal chips had a smaller heat capacity than the solid metal.
However, Rumford disposed of this notion by measuring the specific heat capacities of both chippings and solid metal. They both proved to be equal, and hence the overspill of caloric could not have resulted from a change in the heat capacity of the brass.
He placed a brass cylinder in a wooden box filled with water and then subjected it to the action of a blunt borer worked by horses. As the operation proceeded the water gradually warmed up until, after about 165 minutes, it actually boiled.
Obviously, caloric could not have come from the water, since this had gained caloric.
As he saw the situation, motion only had been supplied to the brass cylinder and borer.
He therefore affirmed his belief in the older idea that heat was a form of motion.
Heat as a form of energy
During the years which followed Rumford’s observations the concept of energy or work began to emerge in scientific thought.
Moreover, the rapid development of steam power, which was then taking place, played its part in stimulating a general interest in the relation between heat and mechanical work.
In England, Thomas Young drew attention to the confusion which existed between force as such and the work which is done when a force is exerted through a distance.
He was also the first to use the word energy to refer to the capacity of a moving body for doing work.
The German physicist, Robert Mayer, calculated the work done when a gas is compressed and assumed that the whole of this work became converted into internal energy which increased the temperature of the gas.
Eventually, however, during the period 1840-50, a series of experiments was carried out by James Joule which settle the problem of the nature of heat beyond all reasonable doubt.
Joule’s work on the relation between thermal units of heat and mechanical units of energy
James Joule spent many years making careful experiments to show that mechanical, electrical and chemical energy could be converted into heat, and his great achievement was to measure the number of mechanical units of work which, when converted into internal molecular energy in a substance, produced the same temperature rise as one thermal unit of heat.
Joule’s experiment
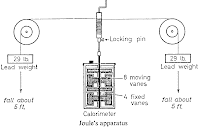 |
| Joule’s apparatus |
One of the most famous of Joule’s experiments is illustrated in the picture from which it will be seen that he worked in Imperial ( English) gravitational units.
The name calorimeter is given to any vessel or piece of apparatus in which heat measurements are carried out.
Inside the calorimeter were four fixed vanes which prevented the water from being carried round bodily. Thorough churning of the water thus took place.
By means of a handle the weights could be wound up and allowed to fall several times.
The kinetic energy of the paddle then becomes transformed into internal molecular energy in the calorimeter and its contents, with an accompanying rise in temperature.
Joule’s calculation
From the measured rise in temperature and previous measurement of heat capacity of the calorimeter and its contents Joule calculated the heat energy in thermal units which would produce the same temperature rise.
The heat in thermal units was then equated to the work done in mechanical units, and from this the work equivalent to one thermal unit of heat was found.
As we have seen, Joule worked in English units, as the metric system had not then come into general use. When converted into present-day SI units, the final results he optained was,
1 calorie = 4.2 joules
1 kilocalorie = 4200 joules
