Conversion of potential energy into internal energy
In accordance with the principle he was trying to establish, Joule came to the conclusion that the water at the bottom of a waterfall ought to be slightly warmer than that at the top.
 |
| Waterfall |
At the top of the fall the water possesses ( yes, 5 s 🙂 potential energy, which becomes converted to kinetic energy as it descends. Part of this kinetic energy becomes transformed into internal molecular energy when the motion of the water is arrested at the bottom.
Joule decided to carry out a test on a particular waterfall in Switzerland. Although the expected temperature rise was exceedingly small, he was able to detect it with a very sensitive thermometer and found the result to agree closely with the calculated value. This man was very clever.
Experiment to measure the number of Joules equivalent to a calorie by the shot-tube method.
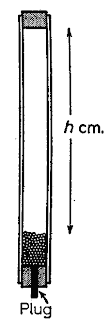 |
| Heat by falling shot tube |
This experiment illustrates the conversion of potential energy into internal molecular energy, and may be used to find a rough value for the number of joules equivalent to a calorie.
A cardboard tube about a metre long contains a quantity, m in kg, of lead shot and is fitted with corks at both ends. One of the corks has a small hole plugged with a wooden peg. This allows for the insertion of a thermometer to take the temperature of the shot.
If the tube is inverted, the shot falls through a distance h in m. In this so doing its potential energy, mgh in J, becomes transformed into kinetic energy which, in turn, becomes internal molecular energy when the shot is brought to rest.
The temperature, Θ1, of the shot is taken immediately before the experiment starts and the tube is then inverted 100 times in order to obtain a measurable temperature rise. The final temperature, Θ2, of the shot is noted.
The results of the experiment
Assuming, specific heat capacity of lead = c = 30 cal/ kg °C ( thermal units)
and g = 9.8 m/ s²
.
The number of joules equivalent to a calorie may be calculated as follows:
Force in newtons on lead in falling = mg
Total distance fallen in metres = 100 × h
Work done in joules = 100 mgh
Rise in temperature of lead = ( Θ2 – Θ1) = Θ
Equivalent heat input in calories = mcΘ
Hence, number of joules equivalent to 1 cal = Work in joules / heat in calories
= 100 mgh / mcΘ
= 100 gh / cΘ in joules/ calorie
.
Note that the mass of the lead disappeared from the last final equation, and therefore the shot does not have to be weighed.This experiment is worth trying as a matter of interest, but you are warned not to expect a very accurate result.
There are two main sources of error. First,
the whole of the shot does not fall through h, as some of it inevitably beings to slide before the tube reaches a vertical position.
Secondly,
the shot cools in falling through the air. The air thus gains some of the internal energy produced, and this, in turn, is lost to the cardboard.
Temperature rise resulting from compression
Anyone who has pumped up a bicycle tyre knows that the lower part of the pump barrel may become quite warm.
Erroneously,this is often attributed to work done against friction. On reflection, however, one must come to the conclusion that the friction of an oiled plunger against the smooth barrel wall is far too small to do any appreciable amount of work. The increase in internal energy which raises the temperature comes, of course, from the work done in compressing the air.
Consequently, if compressed air or any other gas is allowed to expand it performs external work, and the energy required comes from the internal energy of the gas itself. Consequently, the gas cools.
Importance of Joule’s work
When an account of Joule’s work becomes known in the middle of the nineteenth century it aroused but little interest, as the concept of work and energy was new to science. At the time it was not generally realized that Joule’s experiments provided the first reliable experimental evidence for the truth of the principle of the conservation of energy.
This principle was put forward by the German physicist, Herman von Helmholtz, in a book published in 1847, but it had earlier been accepted by other far-seeing scientists, particularly Sadi Carnot, Robert Mayer and Sir William Grove.
Joule’s experiments had shown that internal molecular energy could be put into a substance either by heat or by mechanical work and that there was an exact equivalence between those two forms of energy. Later it was demonstrated that the same exact relationship existed between other forms of energy, for example, electric energy, chemical energy and heat. One can readily appreciate why Joule’s memory has been honored by giving his name to the SI unit of energy.
Once the principle of conservation of energy had thus been established, the way became open for great advance in science. It formed the basis of a new branch of the study of heat and energy known as thermodynamics. Calculations could now be made regarding certain problems in pure science with a certainty that the answer would be correct. In the field of applied physics the same can be said with regard to calculations on the design of steam engines, internal combustion engines, rockets and jet engines, electric motors, generators and power installations.
The first law of thermodynamics
The work of Joule and others may be summed up in a statement known as the first law of thermodynamics:
change in internal energy of any system = heat inflow + work done on the system
or, in symbols U = Q + W
This is simply the way of stating the law of conservation of energy as applied to heat and mechanical work changes. The equation must, of course, be treated algebraically, i.e., heat outflow and work done by the system would be written with minus signs.
The word system in the above equation refers to any body or device which is involved with heat, work and energy changes. The steam engines turbine can be taken as a practical example of such.
Summary
1- The expression heat energy in a body should be avoided and the term internal energy used instead. Otherwise there is a danger of confusing heat with internal energy. As we have already explained, heat is the name given to the process of energy transfer from one body to another caused by a temperature difference between them.
2- Heat is only one way of transferring energy to a body. We have seen that energy can also be given to a body by a force moving through a distance, in which case the process of energy transfer is called work
3- Work done on a body may or may not change its internal energy. For example, a frictional force acting through a distance on the body will do work which is converted into internal molecular energy and this will have the same effect as heat transfer, i.e., it will produce a rise in temperature.
4- By contrast, the work done by a force in lifting a body above the earth’s surface will be converted into gravitational potential energy of the body as a whole without affecting the internal energy of its molecules.
I hope you have found this post useful.


