14–5 SECONDARY CELLS
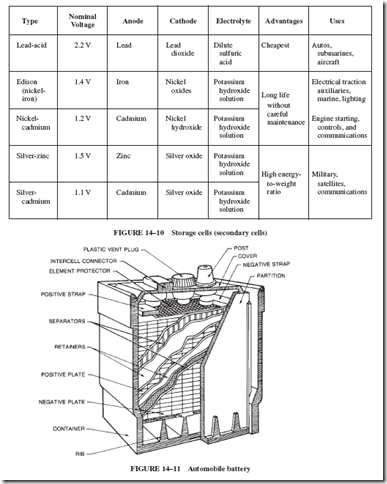
In one family of cells, the electrochemical action is reversible; that is, if the discharged cell is connected to another electron source so that electrons can be put back into the negative terminal of the cell, the cell is recharged to its original emf value. Cells of this type are called storage cells or secondary cells. Figure 14–10 lists five types of secondary cells in order of increasing cost.
The Lead Storage Battery
Each lead cell produces 2 volts. Automobile batteries rated at 12 volts contain six cells connected in series; see Figure 14–11.
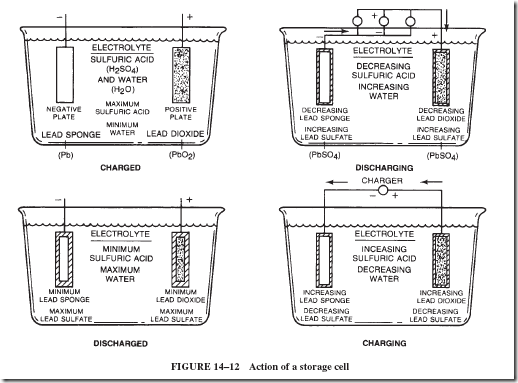
The action of a storage cell is shown in Figure 14–12. The negative plate consists of metallic lead. When the cell is producing current, lead atoms on the surface of the plate lose two electrons each to become Pb++ ions. These Pb++ ions do not dissolve into the electrolyte but remain on the plate and attract SO422 ions from the sulfuric acid solution. As a result, an invisible thin layer of PbSO4 is formed on the negative lead plate.
The positive plate consists of lead dioxide, PbO2. Each lead particle in the plate lacks four electrons (these electrons were given to the oxygen when the compound PbO2 was formed). Each Pb ++++ ion takes two electrons from the external circuit to become a Pb++ ion.
The energy for the electron transfer results from the tendency of neutral lead atoms to give two electrons each to Pb++++ ions, so that both Pb++++ ions and Pb atoms become Pb++ ions.
When each Pb++++ ion of the lead dioxide takes up two electrons, the ion can no longer hold the oxygen. The oxygen, therefore, goes into the acid solution and combines with the hydrogen ions of the acid to form water molecules. The Pb11 remains on the plate and picks up SO4– from the sulfuric acid to form lead sulfate.
The chemical action in the lead storage battery can occur only where the plates are in contact with the sulfuric acid solution. If a large current is required, the plates are
constructed so that the surface area in contact with the electrolyte solution is large. In a cell, the plates are arranged as shown in Figure 14–13. As indicated in Figure 14–12, the negative plate is made of lead sponge and the positive plate of porous lead dioxide. The porosity of these plates makes it possible for a large surface area of material to be wet by the electrolyte. Separators of wood, glass fibers, or similar porous material keep the plates from touching each other. To provide mechanical strength, both the negative and positive plates consist of an open framework of an alloy of lead-antimony. The active material is pressed into this framework. The electrolyte is dilute sulfuric acid having a specific gravity of 1.28.
Batteries can be shipped wet (filled with the electrolyte) or dry (with the electrolyte packaged in a separate container). The relative packing and shipping costs determine which shipment method is used.
The Ampere-Hour Rating
An ampere-hour is the amount of charge delivered by 1 ampere in 1 hour (1 ampere- hour 5 3,600 coulombs). The ampere-hour rating of a battery is determined from its measured ability to produce current for 20 hours at 80°F; therefore, a battery that produces 6 amperes steadily for 20 hours at 80°F has a rating of 120 ampere-hours. If the discharge rate of the battery is 1 or 2 amperes instead of 6 amperes, a 120-ampere-hour battery can produce more than 120 ampere-hours. The battery cannot produce 120 amperes for 1 hour; the actual value of ampere-hours produced depends on the current.
Battery Charging
A rectifier or DC generator is required to charge a battery. The battery is charged by forcing a current through it in a direction opposite to that of normal battery operation. In the lead cell, this reversal of current reverses the chemical changes that take place in the cell when it furnishes energy. (Recall that in primary cells, the chemical changes cannot be reversed.)
The charging process in the lead cell is a series of chemical reactions. Recall that a current through a solution of sulfuric acid in water produces hydrogen at the negative plate and oxygen at the positive plate. If the plates are already covered with a thin layer of lead sulfate (from the discharging process), then the H1 ions forced toward the
negative plate combine with the SO — ions to re-form sulfuric acid. The electrons forced onto the negative plate by the generator combine with the Pb++ ions to form lead atoms, as shown in Figure 14–14A.
At the same time, water decomposes at the positive plate. The hydrogen set free by this process combines with the SO x–ns on the plate to form more sulfuric acid. The oxygen resulting from the breakdown of the water combines with the lead to form lead dioxide. The Pb11 ions of the discharged positive plate are converted to Pb++++s as the generator removes electrons; see Figure 14–14B.
The following equation summarizes the charge/discharge process of the battery.
In a charging circuit, such as the one shown in Figure 14–14, the generator voltage must (1) equalize and overcome the battery emf, and (2) have a large enough value to produce a current through the resistance of the circuit.
A charging rate that is too high damages a battery by causing it to overheat. It can also cause gas bubble formation inside the spongy plate material, which forces active material to break away from the plate structure. The safest charging procedure is a 10-ampere rate, or less, requiring about 24 hours. A battery can be charged on a constant voltage circuit in 6 or 8 hours, starting with a 30-ampere or 40-ampere rate, which tapers down as the battery charge builds up. With this method, however, the battery should be checked to see that it is not overheating. The accepted limit is usually 110°F.
Battery Testing
A hydrometer measures the specific gravity of the electrolyte in each cell. Specific gravity is the ratio of the weight of a given volume of a liquid to the weight of an equal volume of water. For example, a sample of pure acid may have a specific gravity of 2. Since the specific gravity of water is defined as 1, it follows that, volume for volume, the acid weighs twice as much as water. A charged battery has enough sulfuric acid in the electrolyte so that its specific gravity is 1.25 to 1.28 (water 5 1.00). As the battery discharges, SO4 ions of the acid are tied up on the plates, and the electrolyte becomes more like plain water, its specific gravity approaching 1.1. Since occasionally a cell may not operate properly even when it has sufficient acid, a better method of battery testing measures the voltage of each cell when it is producing current. Voltage of a good cell will remain at 2 volts even though the cell is producing 20 or 30 amperes. Voltage of a dead cell will be 2 volts when the cell is not producing current but will drop off when it is producing current.
Hydrometer calibrations normally omit the decimal point. For example, the reading for a fully charged cell will be from 1250 to 1280, which is understood to mean the specific gravity is equal to 1.25 to 1.28. The indication for a completely discharged cell will be about 1100 (meaning specific gravity equals 1.1).
Battery Care
Particular care should be taken to avoid getting dirt into a cell. When the cap is re- moved, it should be set in a clean place, if it has to be set down at all. Dirt, especially a few flakes of iron rust, can spoil a cell permanently. A great many automobile batteries probably go bad because of the accidental entry of dirt into a cell.
A battery should not be allowed to remain in a discharged condition. If a battery is completely run down, it should be charged within a few hours at a slow rate. If the discharged battery is allowed to stand discharged, the lead sulfate apparently hardens or crystallizes and is difficult to restore to lead and lead dioxide. Also, the watery solution in a discharged battery can freeze in winter, cracking the battery.
The liquid should be maintained at a level that covers the plates. Distilled water is preferable, but faucet water is better than none at all. (Melted frost from the refrigerator is distilled water.) Water is lost from a battery mainly by evaporation or slightly by hydrogen and oxygen forming during charging. Acid is lost only by cracking the case or tipping it over, so acid seldom is needed.
There is no point in adding various amazing powders and liquids to a battery to improve its performance, life, and complexion. For many, many years it has been known that Epsom salt (MgSO4) or sodium sulfate (Na2SO4) can be added to a lead storage cell electrolyte in small amounts without damage to the battery but without doing it any good either. If acid has been lost from the cell, these materials provide ions that can be useful. But a teaspoonful of sulfuric acid (worth $0.02, like the Epsom salt) is more desirable in such a battery. When new and important discoveries appear, more reliable remedies will be announced in news articles and electrical engineering journals rather than in exclamatory and sensational advertising.