Electrostatics
3–1 STATIONARY ELECTRONS
The word static means that something is at rest; it is not going anywhere. There- fore, static electricity is a charge that is stationary; it is not a current.
A good deal can be learned about electrons when they are stationary. Even though they are not traveling, electrons do not sit still but constantly jump from one place to another. The sparks and arcs they produce are often very important.
The word static in a discussion of electricity may first remind one of noise in a radio. When lightning flashes or an electric spark occurs, vibrating electrons broadcast some energy that is received as crackling in a radio receiver. The term static is now applied to any unexplained radio interference, whether the interference is of true electro- static origin or not.
The material covered in this chapter is an extension of ideas introduced in Chapter 1. The Summary of Chapter 1 should be reviewed at this point, particularly these useful facts:
• Any object that has a negative charge has received some electrons that normally do not belong to it, and they will try to escape.
• Any object that has a positive charge has lost some of its electrons. When a positively charged object has electrons returned to it, it goes back to its original neutral state. The returned electrons do not have to be the same ones that were lost. (Remember that electrons are all alike, regardless of where they come from.) If a million electrons are lost and a different million electrons return, everything is still back to neutral.
Lists A and B in Figure 1–1 introduced insulators (nonconductors). Insulators easily acquire a static electric charge, because they are materials in which electrons cannot move readily. If a spot on the insulating material has a surplus of electrons, it keeps the electrons. If at another location on the material there is a lack of electrons, however, that surplus finds it difficult to slide over and fill the lack.
Materials in which electrons move freely are called conductors. Metals, with their loose electrons in the outer rings of the atoms, are good conductors. Conductors can be charged only if they are insulated from their surroundings by some nonconducting material.
3–2 ELECTROSCOPES
The electroscope was once widely used to compare electrical charges and served as a crude voltmeter. It was also used in the detection of radioactive minerals. The device was an important aid in experiments that led to the discovery of electrons. For present-day use, it has been replaced by devices that are more accurate and sensitive but that are also more complex.
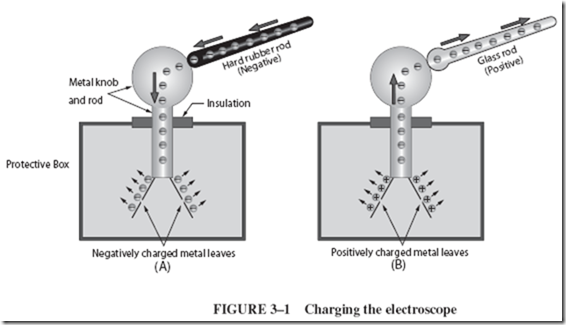
The electroscope, Figure 3–1, must first be charged using a known charge. The charging procedure is simple: (1) A hard rubber rod is charged by rubbing it on wool; (2) then the hard rubber rod is slid along the knob of the electroscope. Electrons from the negative hard rubber are transferred from the rod to the electroscope, where they repel each other and scatter all over the metal knob, metal rod, and metal leaves. The leaves repel each other because both are charged alike, in this case, negatively, as shown in Figure 3–1A.
The electroscope can also be charged positively, using a charged glass rod instead of the hard rubber rod. As the glass rod is wiped along the knob of a neutral electroscope, the rod attracts electrons from the electroscope. The electroscope now has a lack of electrons (since they have been transferred to the glass rod), and the electroscope is positively charged. The leaves again spread apart, as shown in Figure 3–1B, because the like positive charges repel.
Unknown Charges
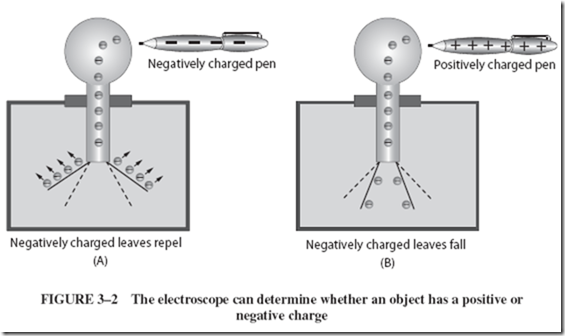
A charged electroscope will indicate whether an unknown charge is positive or negative. For example, a pen is charged by rubbing it on a sleeve. The question now is whether the pen is negatively or positively charged. With a hard rubber rod, the electro- scope is charged negatively, as described before. As shown in Figure 3–2A, the pen is then brought slowly toward the top of the electroscope, and the leaves are observed closely for their motion. The leaves may repel even more. Why?
The leaves were made negative with the rubber rod. If they repel more, they must now be more negative. The leaves can become more negative only by having more electrons forced onto them. The unknown charge on the pen, therefore, must be negative, because more of the electroscope’s electrons were repelled to the leaves.
If the pen is charged positively, as in Figure 3–2B, what will happen? The positively charged pen will attract some of the excess electrons from the leaves to the knob. As these repelling electrons are removed, the leaves will fall together.
A positively charged electroscope can also be used to identify unknown charges. Keep in mind that the positively charged electroscope has relatively few of its electrons removed; there are still many electrons there. When a negative object comes near, it will repel the electrons in the electroscope toward the leaves. These electrons will neutralize the repelling positive charges on the leaves, causing the leaves to fall together. An approaching positive object will pull even more electrons from the positively charged leaves, causing them to repel each other more strongly.
In these instances, all of the electron motion is within the electroscope itself. The charged pen is not brought close enough to the knob to permit electrons to jump the gap between the two objects.
3–3 ELECTROSTATIC INDUCTION
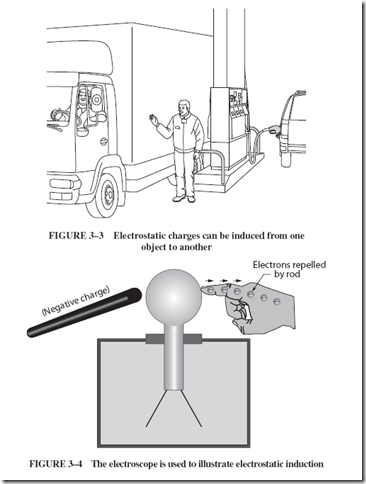
A charged object always affects other objects in its neighborhood either by at- tracting or repelling electrons within the nearby object. Electrostatic induction is the process by which the neighboring object acquires a charge. In unusual circumstances, electrostatic induction can cause accidents. For example, on a warm, dry day in south- ern Oregon (where there are no self-serve gas stations), a truck driver speaks to the new gas station attendant, Joe, as shown in Figure 3–3. Joe lets go of the iron post he was sitting on near the gas pump. As the truck drives away, he steps over to remove the hose from another customer’s gas tank. At this point, the gasoline catches fire. What causes this to happen?
A strongly charged truck, Joe’s new rubber-soled shoes, the removal of his hand from the grounded post, electrostatic induction, and a spark in the presence of gasoline fumes all contributed to the fire. Because the truck was negatively charged, it repelled electrons from Joe through the post to ground (earth). Having lost some electrons, Joe was then positively charged. When he removed his hand from the post, Joe did not immediately regain the lost electrons since he was insulated by his rubber-soled shoes and the dry air. Electrons, attracted to his positive hand from the gasoline tank, jumped the gap, causing a spark. The spark ignited the gasoline vapor.
Let us illustrate this electrostatic induction process using the electroscope shown in Figure 3–4.
1. Bring a negatively charged rod (truck) near the electroscope (Joe). The experimenter’s hand touching the electroscope corresponds to Joe’s connection to the ground through the post. The rod will repel electrons from the electroscope knob. These electrons will move from the electroscope to the experimenter’s hand touching the knob.
2. Remove the hand from the knob. This leaves the electroscope with a positive charge (lack of electrons).
3. Remove the negative rod.
If these steps are performed in the correct order, the electroscope will be left in a charged condition, shown by its repelling leaves. The fact that the charge is positive can be checked by its behavior when the negative rod is slowly brought near it again. The charge on the electroscope and the charge on Joe are examples of induced charges.
An induced charge on one object is caused by the approach of another charged object, without contact between the two objects.
Benjamin Franklin showed that lightning is a large-scale performance of ordinary electrostatic behavior. (A few people who tried his kite experiment were killed by it.)
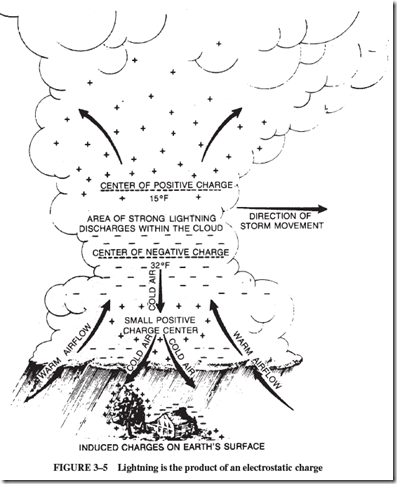
The distribution of electrical charges in thunderclouds has been mapped, but as yet no one knows just how the charges are formed. Most of the lightning flashes occur within the cloud itself. Whether electrons go from cloud to ground or from ground to cloud depends on the part of the storm area that is over a particular location; see Figure 3–5.
Objects, such as a house, that are under the small, positively charged area at the center of the storm are hit by electrons moving from the earth to the cloud. Objects, such as trees, under the large, negatively charged part of the cloud are hit by electrons driving from the cloud to the earth.
It is not possible to insulate against lightning. Protection can be provided only by giving it an easy path to ground. Well-grounded lightning rods are very effective. Ammunition sheds have been protected simply by a steel cable supported along the ridge of the shed and grounded at each end. Steel frames of large buildings, grounded during construction, serve as lightning protection for the building.
An automobile may be struck by lightning, but its occupants are not harmed because the steel body conducts the high-voltage electrons away from them. (Crawl- ing under the car is definitely unsafe!) A lightning arrester for TV and radio antennas is simply a grounded wire brought close to, but not touching, the antenna. Lightning arresters for power lines are made of materials with specialized resistance properties. The materials permit the high-voltage lightning discharge to pass to ground but stop the lower- voltage energy of the power line from being grounded.
3–5 NUISANCE STATIC CHARGES
Besides lightning and the radio interference it causes, static charges are responsible for a variety of other nuisances and hazards.
• Power belting readily becomes charged. Grounded pulleys, combs or tinsel bars close to the belt, conductive belt dressing, and the use of conductive belt materials help pre- vent the buildup of static electricity.
• Charges that have accumulated on trucks and cars are usually grounded by a wire on the appro chway to a tollgate to prevent electrical discharges (shocks) when coins change hands.
• Anesthetic gases are combustible. Precautions are taken to avoid static sparks, which may cause explosions during surgical operations. Grounded equipment, moist air, and conductive rubber help prevent the accumulation of charges.
• Grain dust, flour, wood dust, and cotton lint have produced disastrous explosions. Prevention of static charges helps avoid such dust explosions.
• In printing, sheets of paper may fail to feed into the press or to stack properly if they repel each other or are attracted to nearby objects. When printing is done from a continuous sheet, the sheet may become charged and ignite if combustible solvent vapors are present. Similar problems occur in the cloth and plastic industries. Some devices that ground or neutralize these charges are the tinsel bar, flame, or a long metal comb connected to an alternating current power supply.
3–6 USEFUL STATIC CHARGES
Painting
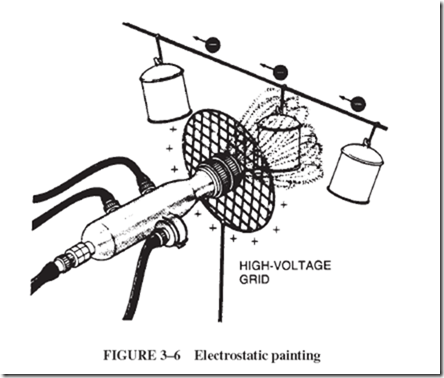
Although some industries must prevent it, other industries use the static charge as a tool. In paint-spraying applications, paint particles, given a charge after they leave the spray gun, are attracted to the oppositely charged object receiving the paint, as illustrated in Figure 3–6. This method produces an even coat without wasting paint, and paint particles
do not accumulate in holes or openings since the charge there is no greater than that at the outer surface.
Sandpaper Manufacture
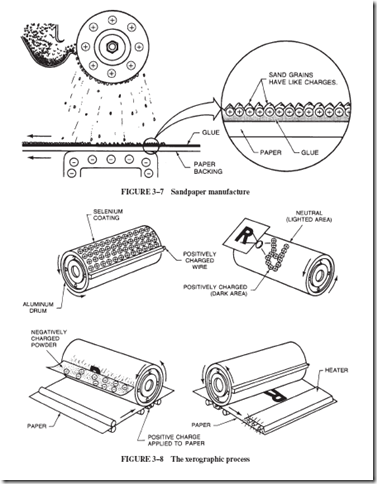
Sandpaper grit can be made to stand up as it is being applied to the paper backing by giving the sand particles a positive charge and the glue-covered paper backing a negative charge; see Figure 3–7. Since the individual sand grains have like charges, they repel each other and stand apart. This method produces a sharper sandpaper.
Smoke Precipitators; Rug Manufacture
Smoke precipitators charge the smoke particles, which are then collected on oppositely charged screens, so that the particles are not released to pollute the atmosphere. Similarly, charged rug fibers are attracted to oppositely charged, glue-covered backings to form new types of rugs and fabrics.
Xerography (Dry Copying)
Chester F. Carlson (1906–1968), the inventor of the process known as xerography, or dry copying, had worked for nearly 15 years on the application of electrostatic charges for office copiers before such machines appeared on the market around 1950. The core of the machine is a rotating aluminum drum coated with a thin film of the element selenium, a semiconducting material; see Figure 3–8. The conductivity of selenium is increased by light. The machine operates in the following manner:
1. As the selenium surface rotates past a positively charged wire, it loses electrons to the wire. This charging of the selenium is done in darkness.
2. Light, reflected from the sheet of material to be copied, is projected by lenses and mirrors onto the positively charged selenium on the drum. The black form of the letter R, for example, reflects no light to the drum. However, the white paper back- ground does reflect. This reflection forms an image there, just as a camera or projector forms an image. Where the light surrounding the letter strikes the selenium, the selenium becomes a better conductor. Electrons flow from the aluminum drum to this lighted area and neutralize its positive charge. The dark area of the drum, where the letter R was, remains positively charged.
3. A small conveyor belt, not shown in Figure 3–8, pours a mixture of positively charged glass beads and negatively charged black powder over the previously illuminated surface of the drum. The black particles are attracted to the positively charged areas left on the drum in Step 2. The black particles are not attracted to the neutralized areas of the polished selenium surface. Any excess black-powder toner and glass beads fall away.
4. The piece of paper that is to receive the finished print now passes under the drum.
The paper has been positively charged by a wire so that it attracts the negatively charged black powder from the surface of the drum.
5. Finally, the paper carrying the black-powder image passes either under a radiant heater or over a heated roller, where the black particles are melted into the paper to form a permanent copy. A brush (not shown) removes any particles still on the drum, and the selenium surface is ready for recharging.
Electrostatic Generators
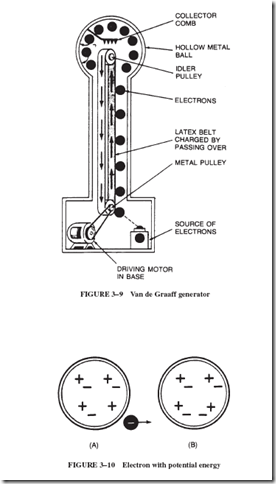
Many types of static generators constructed in the past produced tiny currents at high voltage. A much more efficient device is the Van de Graaff generator, shown in Figure 3–9, developed by the American physicist Robert J. Van de Graaff (1901–1967). This device is used to create high voltages for speeding up charged particles in atom-smashing experiments. This generator can also be used to test lightning protection equipment. Small models of the Van de Graaff generator are available for small-scale laboratory work.
In Figure 3–9, the sphere at the top of the device charges to a few hundred thousand volts, but the number of electrons accumulated there is small enough so that the spark is harmless.
3–7 POTENTIAL ENERGY OF ELECTRONS
An electron forcibly taken away from one neutral object and put on another neutral object has gained potential energy; see Figure 3–10. Force is needed to pull the electron away from Object A because the electron is attracted back to A by the positive charge that remains on A. If several electrons are transferred to Object B, they repel additional electrons from A. These electrons possess potential energy. Given the opportunity, the electrons will return to A, producing heat as they return. If these electrons are permitted to return to A through an electric motor, their potential energy is converted to mechanical energy.
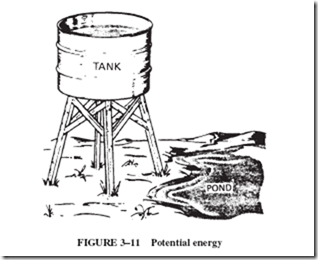
This concept may be compared to another example of potential energy, as illustrated in Figure 3–11. Water can be taken from the pond and put into the tank, but it cannot get there by itself. In other words, energy must be used to carry the water up to the tank. The water, however, then has potential energy. Given the opportunity, the water in the tank will run back down to the ground, releasing energy on whatever it hits; that is, it can do useful work if it passes over a water wheel or drives a water turbine.
The energy used to carry the pond water up to the tank is not wasted. Each gallon of water in the tank has more potential energy than a gallon of water in the pond. There is a potential energy difference between the water in the tank and the water in the pond.
In a similar manner, there is a potential energy difference between the electrons in Object B in Figure 3–10 and the electrons in Object A. In other words, there is a potential difference between B and A. It is precisely this difference in potential energy that is expressed by the word voltage, a very common term in our electrical vocabulary that is more fully explained in Section 4–2.
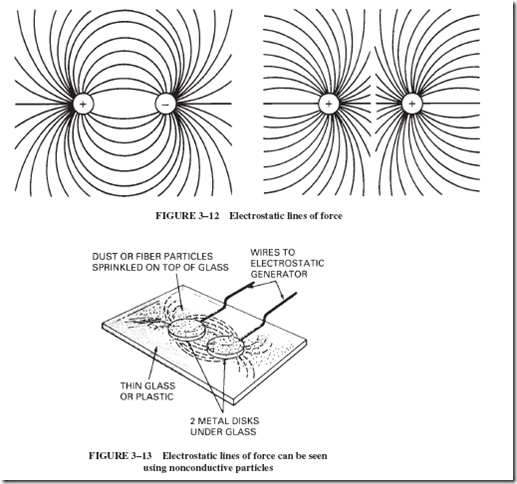
3–8 ELECTROSTATIC LINES OF FORCE
Many years ago, people were puzzled as to just how two objects could exert force on each other when they were some distance apart with no material connection between them. How do two oppositely charged objects attract each other in a vacuum in the absence of light and heat? Even today, this question is still puzzling. We have no evidence that any small particles pass back and forth between them to pull them together or push them apart. There is no evidence that any sort of wave passes from one object to another.
Years ago, a solution to this puzzle was proposed by picturing invisible lines of force, like ropes or rubber bands, pulling two opposite charges together, as shown in Figure 3–12. The pattern of these lines may actually be seen by scattering splinters or dust of some non- conducting material between two strongly charged objects, as illustrated by Figure 3–13. Shredded wheat, short fibers, grass seed, or wood splinters can be used. The short fibers become charged by induction and tend to become aligned in patterns like those shown in Figure 3–12. These patterns, referred to as electrostatic lines of force, show the existence of a very real force between two electrically charged objects. Later, as you proceed with your studies, you will learn that similar force fields exist between magnetic poles as well
(see Chapter 15). The graphic representation of such a magnetic force field may look similar, but it is a distinctly different subject. Do not confuse one with the other.
SUMMARY
• A negatively charged object has a surplus of electrons; a positively charged object lacks some electrons.
• A charged electroscope identifies unknown charges.
• Objects may become charged by being near a charged object, without contact. This is known as electrostatic induction.
• Potential difference is measured in volts. It is the energy difference between electrons in different locations.
• Charged objects are surrounded by an electric field that consists of electrostatic lines of force extending from the charged object.
Achievement Review
1. What is static electricity?
2. Is static electricity more often noticed on conductors or insulators? Why?
3. Can conductors be charged?
4. Describe what happens when a positively charged object comes near but does not touch a positively charged electroscope.
5. What happens when a negatively charged object comes near a positively charged electroscope?
6. What is electrostatic induction?
7. When a negatively charged object approaches a neutral object, what sort of charge is induced on the neutral object?
8. Charged objects are said to possess energy. Explain.
9. What are electrostatic lines of force?
10. How does a lightning arrester stop lightning?
11. State a few industrial uses of static charge.
12. What industries try to avoid static charges?