CORROSION
There are four basic causes of corrosion:
• Corrosive acids
• Oxygen
• Galvanic action
• Biological organisms
Corrosive Acids
Aggressive or strong acids, such as sulfurous, sulfuric, hydrochloric, and nitric, are found in most industrial areas. These acids are formed when certain industrial-waste gases are washed out of the atmosphere by water showering through a cooling tower. The presence of any of these acids will cause a drop in circulating- water pH. Water and carbon dioxide are found everywhere. When carbon dioxide is dissolved in water, carbonic acid is formed. This acid is less aggressive than the acids already mentioned. Because it is always present, however, serious damage to equipment can result.
Oxygen
Corrosion by oxygen is another problem. Water that is sprayed into the air picks up oxygen. This oxygen then is carried into the system. Oxygen reacts with any iron in equipment. It forms iron oxide, which is a porous material. Flaking or blistering of oxidized metal allows corrosion of the freshly exposed metal. Blistering also restricts water flow and reduces heat transfer. Reaction rates between oxygen and iron increase rapidly as temperatures increase. Thus, the most severe corrosion takes place in hot areas of equipment with iron parts.
Oxygen also affects copper and zinc. Zinc is the outer coating of galvanized material. Here, damage is much less severe because oxidation of zinc and copper forms an inert metal oxide. This sets up a protective film between the metal and the attacking oxygen.
Galvanic Action
Galvanic corrosion is the third cause of corrosion. Galvanic corrosion is basically a reaction between two different metals in electrical contact. This reaction is both electrical and chemical in nature. The following three conditions are necessary to produce galvanic action:
• Two dissimilar metals possessing different electrochemical properties must be present
• An electrolyte, a solution through which an electrical current can flow, must be present
• An electron path to connect these two metals is also required
Many different metals are used to fabricate air-conditioning and refrigeration systems. Copper and iron are two dissimilar metals. Add a solution containing ions, and an electrolyte is produced. Unless the two metals are placed in contact, no galvanic action will take place. A coupling is made when two dissimilar met- als, such as iron and copper, are brought into contact with each other. This sets up an electrical path or a path for electron movement and allows electrons to pass from the copper to the iron. As current leaves the iron and reenters the solution to return to the copper, corrosion of the iron takes place. Copper-iron connections are common in cooling systems.
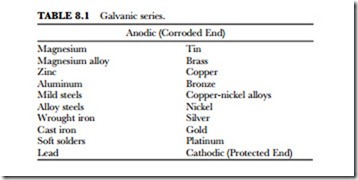
Greater separation of metals in the galvanic series results in their increased tendency to corrode. For ex- ample, if platinum is joined with magnesium with a proper electrolyte, then platinum would be protected and magnesium would corrode. Since they are so far apart on the scale, the corrosion would be rapid (see Table 8.1). If iron and copper are joined, we can tell by their relative positions in the series that iron would corrode, but to a lesser degree than the magnesium mentioned in the previous example. Nevertheless, corrosion would be extensive enough to be very damaging. However, if copper and silver were joined together, the copper would corrode. Consequently, the degree of corrosion is determined by the relative positions of the two metals in the galvanic series.
Improperly grounded electrical equipment or poor insulation can also initiate or accelerate galvanic action. Stray electrical currents cause a similar type of corrosion, usually referred to as electrolytic corrosion. This generally results in the formation of deep pits in metal surfaces.
Biological Organisms
Another cause of corrosion is biological organisms. These are algae, slime, and fungi. Slimes thrive in com- plete absence of light. Some slimes cling to pipes and will actually digest iron. This localized attack results in the formation of small pits, which, over a period of time, will expand to form holes.
Other slimes live on mineral impurities, especially sulfates, in water. When doing so, they give off hydro- gen-sulfide gas. The gas forms weak hydrosulfuric acid. (Do not confuse this with strong sulfuric acid.) This acid slowly but steadily deteriorates pipes and other metal parts of the system. Slime and algae release oxy- gen into the water. Small oxygen bubbles form and cling to pipes. This oxygen may act in the same manner as a dissimilar metal and cause corrosion by galvanic action. This type of corrosion is commonly referred to as oxygen-cell corrosion.
Algae are a very primitive form of plant life. They are found almost everywhere in the world. The giant Pacific kelp are algae. Pond scums and the green matter that grows in cooling towers are also algae. Live al- gae range in color from yellow, red, and green to brown and gray. Like bacterial slime, they need a wet or moist environment and prefer a temperature between 40 and 80 degrees F (4 and 27 degrees C). Given these conditions, they will find mineral nourishment for growth in virtually any water supply.
Bacteria cause slime. Slime bacteria can grow and reproduce at temperatures from well below freezing (32 degrees F [0 degrees C]) to the temperature of boiling water (212 degrees F [100 degrees C]). However, they prefer temperatures between 40 and 80 degrees F (4 and 27 degrees C). They usually grow in dark places. Some types of slime also grow when exposed to light in cooling towers. The exposure of the dark- growing organisms to daylight will not necessarily stop their growth. The only condition essential to slime propagation is a wet or moist environment.
Fungi are a third biological form of corrosion. Fungi attack and destroy the cellulose fibers of wood. They cause what is known as brown rot or white rot. If fungal decay proceeds unchecked, serious structural dam- age will occur in a tower.
It is essential that a cooling system be kept free of biological growths as well as scale. Fortunately, several effective chemicals are available for controlling algae and slime. Modern algaecides and slimeicides fall into three basic groups: chlorinated phenols (penta-chloro-phenates), quaternary ammonium compounds, and various organo-metallic compounds.
A broad range of slime and algae control agents is required to meet the various conditions that exist in water-cooled equipment. Product selection is dependent on the following:
• The biological organism present
• The extent of the infestation
• The resistance of the existing growths to chemical treatment
• The type and specific location of the equipment to be treated
There is considerable difference of opinion in the trade as to how often algaecides should be added and whether “slug” or continuous feeding is the better method.
In treating heavy biological growths, remember that when these organisms die, they break loose and cir- culate through the system. Large masses can easily block screens, strainers, and condenser tubes. Some pro- vision should be made for preventing them from blocking internal parts of the system. The best way to do this is to remove the thick, heavy growths before adding treatment. The day after treatment is completed, thoroughly drain and flush the system and clean all strainers.
One of the most critical areas of concern about cleanliness is bacteria breeding grounds. The most difficult issue to deal with is stagnant water. A system’s piping should be free of “dead legs,” and tower flow should be maintained. When dirt accumulates in the collection basin of a tower, it provides the right combination of supplies for the creation of Legionella bacteria:
• moisture
• oxygen
• warm water
• food supply
These bacteria can be found in water supplies as well as around rivers and/or streams. They are contained in water droplets and can become airborne. Humans are susceptible to them by breathing in the contaminated air. No chemicals can positively eliminate all bacteria from the water supply in a cooling tower. However, evidence exists to suggest that good maintenance along with comprehensive treatment can dramatically minimize the risk.