Gas Solubility: Henry’s Law
We consider phase equilibrium between a liquid non-ideal mixture with the activity coefficient from the model of the previous section, and an ideal gas vapor phase (fugacity coefficient ϕ// = 1). Then, with the activity coefficient (22.33), Raoult’s law (22.29) assumes the form
where p// = X //p is the partial pressure of α in the vapor.
We consider a not too volatile liquid solvent, say water, under an atmosphere of volatile vapors, say air, where we can expect that the mole fractions of air components (oxygen, nitrogen, argon, …) in the liquid are rather small.
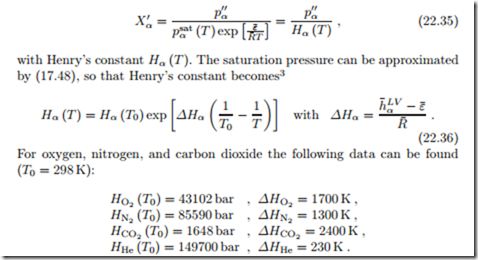
Then, in the above, we can set "’ 1, and find the mole fraction of gases dissolved in the liquid given by Henry’s law (William Henry, 1774-1836),
It should be noted that at environmental temperatures oxygen and nitrogen are well above their critical point, where no saturation pressure exists. There- fore, it is somewhat surprising that the above derivation gives a meaningful result for these temperatures.
In carbonated drinks, carbon dioxide dissolves in water under pressure (normally around 2 bar, depending on temperature). When the pressure is released by opening the bottle or can, the gas bubbles out, to establish the equilibrium for the partial pressure of CO2 at the surface. From (22.36) we see that Henry’s constant grows with temperature, so that less gas is dissolved in warmer water. This is the reason for the effervescence of a warm pop can as compared to a cold one.
Guinness beer is nitrogenated with a special tab. Due to the low dissolution of N2, bottling it would give a very flat beer (under the same pressure one could dissolve 50 times more CO2 than N2), unless a “widget” is used, where N2 is enclosed in a small capsule that releases the gas when the pressure in the can drops after it is opened.
Colder oceans are richer in oxygen, and thus are rich in marine life. There fore big whales migrate between the polar waters where they find most food.
For the solvent, the mole fraction in the liquid is almost unity, which implies that the activity coefficient is close to unity as well, Hence, evaluation of Raoult’s law gives psat (T ) = α, i.e., the partial pressure of water in a saturated water-air mixture is, for all practical applications, equal to the saturation pressure of water, as used in the discussion of psychrometrics.