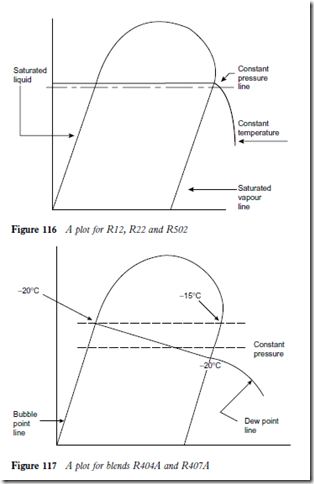
Refrigerant blends
Many refrigerant blends suffer from what is known as ‘glide’. The term boiling point is no longer applicable when dealing with these and instead the term ‘bubble point’ is used.
Briefly the bubble point can be regarded as the temperature at which vapour starts to form at a given pressure. If vaporization continues at the same pres- sure then the temperature at which the last drop of liquid refrigerant vaporizes is known as the ‘dew point’. The dew point therefore is also the temperature at which condensation starts as a superheated vapour is cooled at a constant pressure.
With a pure refrigerant and with a true azeotrope (such as R502) there will not be any temperature changes in an evaporator as the liquid refrigerant vaporizes at constant pressure. With blends it becomes more complicated because the constituents have different volatilities. This means that they will vaporize at different temperatures. The blends do not boil at a constant temperature and pressure. Figures 116 to 118 give examples of the glide when plotted on a pH chart.
A plot for R12, R22 and R502 would be as shown in Figure 116.
A plot for the blends R404A and R407A would be as shown in Figure 117.
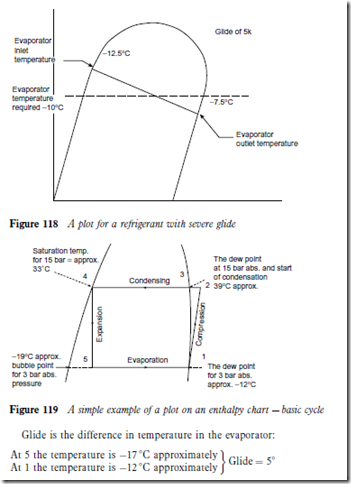
A plot for a refrigerant shows it to have a severe glide. An evaporator would be selected at a mean condition, as shown in Figure 118.
Figure 119 is a simple example of a plot on an enthalpy chart for a basic cycle with no liquid subcooling and no suction superheat. This illustrates bubble point, dew point, boiling range and glide. The cycle is based on 15 bar condensing pressure and 3 bar evaporator pressure.
Boiling range is the difference between dew point and bubble point at a given pressure. For 3 bar the boiling range D -12 – (-19) D 7 °C.
It will be seen from a study of the chart for R404A and R407A that when evaporating at a constant pressure the evaporating temperature rises and this can be as much as a 5K rise. The reverse occurs when condensing.
At constant pressure condensation can commence at 40 °C and finish at 37 °C.