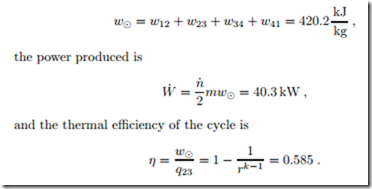
Example: Otto Cycle
As an example we consider an engine operating on the ideal Otto cycle with a compression ratio r = 9. The total swept volume of all cylinders is Vs = 3 litres, and the engine runs at n˙ = 3000 rpm. The intake is at T1 = 300 K, p1 = 0.98 bar, and the heat transfer through the combustion process is q23 = 717 kJ . We determine temperatures, pressures and specific volume for the four corner points of the process, and compute heat and work for all subprocesses. For the computation we treat the working fluid as air with constant specific heats (cold-air approximation), R = 0.287 kJ , cv = 0.717 kJ , k = 1.4.
We begin with the computation of the measurable properties, their numerical values are found in the table below this paragraph. From the ideal gas law we have v1 = RT1 . Since the process 4-1 is isochoric, we have v4 = v1, and the other two volumes follow from the compression ratio as v2 = v3 = v1 . The compression process 1-2 is isentropic, so that p2 = p1rk ; the ideal gas law gives T2 = p2 v2 . The heat for the isochoric heating process (the explosion) is q23 = u3 − u2 = cv (T3 − T2), so that T3 = T2 + q23 ; from the ideal gas law v3 . Since the process 3-4 is isentropic, the pressure at the end of the expansion is p4 = p1r−k ; the temperature follows again from the ideal gas law, T4 = p4 v4 . The numerical values are