Specific, Sensible, and Latent Heat
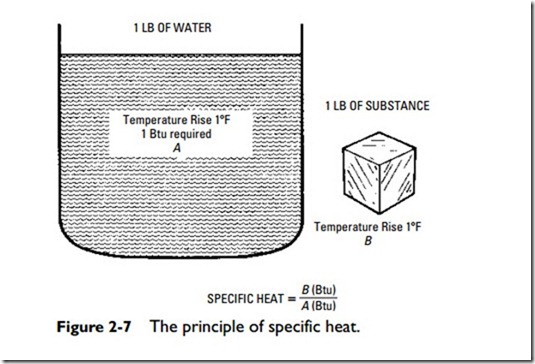
The specific heat of a substance is the ratio of the quantity of heat required to raise its temperature one degree Fahrenheit to the amount required to raise the temperature of the same weight of water one degree Fahrenheit (Figure 2-7). This may be expressed in the following formula:
The standard used in water at approximately 62 to 63oF receives a rating of 1.00 on the specific heat scale. Simply stated, specific heat represents the Btu required to raise the temperature of one pound of a substance one degree Fahrenheit.
Sensible heat is the part of heat that provides temperature change and that can be measured by a thermometer. It is referred to as such because it can be sensed by instruments or touch.
Latent heat is the quantity of heat that disappears or becomes concealed in a body while producing some change in it other than a rise of temperature. Changing a liquid to a gas and a gas to a liquid are both activities involving latent heat. The two types of latent heat are:
1. Internal latent heat
2. External latent heat
These are explained in detail in the next section under Steam.