Secondary lithium ion cells
Rechargeable lithium batteries proved much more difficult to develop than their primary analogues; however, the resultant technology is certainly the most important advance in energy storage in the last fifty years. Lithium, which is one of the lightest elements is also highly electronegative, offering both high potential and capacity. This high electronegativity also necessitates the use of non-aqueous solvents in the electrolyte. To date, lithium metal has not proved suitable for use in rechargeable batteries due to dendrite formation, although work on polymer electrolytes seeks to resolve this problem. Thus, currently available rechargeable lithium batteries tend to use lithium carbon as the negative and metal oxides as the positive electrode. Rechargeable lithium batteries are available in a wide variety of sizes as both lithium ion and lithium polymer (gel type) with most sizes up to about 100Ah currently being available.
Lithium ion batteries
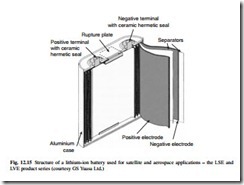
These batteries consist of three active elements, a carbon negative electrode, into which lithium inserts reversibly, a non-aqueous electrolyte immobilized in a porous separator and a positive metal oxide electrode, into also which lithium inserts reversibly, (see Fig. 12.15). The term rocking chair battery has often been used to emphasize the interchange of inserted lithium between the electrodes on cycling. Three forms of
metal oxide dominate commercial batteries, these being layered lithium cobalt oxides, lithium nickel oxides and spinel lithium manganese oxide. The cobalt oxide was the first to achieve commercial success and is still the most widely used. Both nickel and cobalt oxides offer 3.6 V operation in combination with carbon. Although nickel is cheaper than cobalt, its more complex electrochemistry has led to the majority of manufactures preferring cobalt, but lithium manganese oxide is now challenging cobalt oxide for the dominant market position. Although the operating cell voltage is lower at 3.0 V, the considerable advantages in terms of availability, cost and low toxicity more than compensate.
Lithium ion batteries offer high energy density (125 Whkg−1, 300 Whdm−3), high operating voltage (3.6 V), high cyclability with up to 1000 cycles being possible and a rapid recharge (e.g. 2 hours) is possible. There is no memory effect on charge/ discharge as found in nickel cadmium and the batteries are much safer than lithium primary batteries. Self-discharge on standing is less than 10 per cent per month, which is acceptable for a rechargeable battery. Although special high-voltage stable non- aqueous electrolytes have been developed, there are still some issues with stability if careful control of charging is not maintained, and the top-of-charge voltage is exceeded. Charging is carried out at constant current until top-of-charge is reached at about 80 per cent of capacity, then voltage is held constant as the current decays to a limiting value. Normally cells are only sold as part of an integrated pack that contains control electronics and the protection circuit.
Lithium polymer batteries
Considerable efforts have been made to commercialize lithium polymer batteries utilizing a polymer such as polyethylene oxide instead of an organic solvent to dissolve the lithium salt in the electrolyte. Such systems would be able to safely utilize lithium metal as an electrode, which would considerably increase capacity. Unfortunately the electrical resistance of the polymer is still slightly too high for wide-scale commercialization, but some important advances are being made. A slightly different com- pound based on a gel electrolyte has been more successful and most commercial polymer lithium batteries are of this type. Here, a liquid non-aqueous electrolyte is encapsulated in a polymer gel, typically polyvinylidine fluoride or PVDF. Apart from the immobilized electrolyte, such gel-based polymer batteries are very similar to more conventional lithium ion batteries. The polymer construction facilitates a range of innovative concepts based upon winding and folding to the various elements.