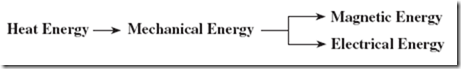|
Electricity Production and Use |
|
2–1 ELECTRICITY PRODUCTION BY ENERGY CONVERSION |
|
As we have seen in Chapter 1, electricity is a form of natural energy and, thus, can neither be created nor destroyed. What mankind has learned, however, is to derive the desired electrical effect from the conversion of any of the existing types of energy, such as heat, light, magnetic, chemical, and mechanical energy. |
|
Sometimes the conversion process is a simple one, producing the desired electricity in a one-step operation. A photocell, for instance, delivers an electrical voltage as soon as light energy falls onto it. |
|
More often than not, the conversion process is a complex one, requiring many intermediate steps. Consider, for example, how large power plants generate electricity for their customers. |
|
The process begins with burning fossil fuels, either coal or gas, to create heat. The heat energy is used to produce steam for a turbine that, in turn, delivers mechanical energy to a generator. Part of this mechanical energy is converted into magnetism because a generator requires magnetic energy to produce the desired electrical energy. The flowchart that follows depicts this multiple conversion process. |
|
Such multiple conversions are rather inefficient if one considers the losses involved in each conversion process. For every dollar’s worth of fuel burnt by the electric power company, only a few cents’ worth of electricity are realized at the consumers’ end. This is because every machine (converter of energy) requires an input that is greater than the output it produces. If you are unfamiliar with this concept, you may want to read about the efficiency of machines in Chapter 19. Remember, every machine (converter of energy) requires more input than it produces output. |
|
Let us now have a closer look at some of the energy sources that are suitable to dislodge the valence electrons from their orbits, thereby creating the desired electrical effect. |
|
2–2 ELECTRICITY FROM FRICTION |
|
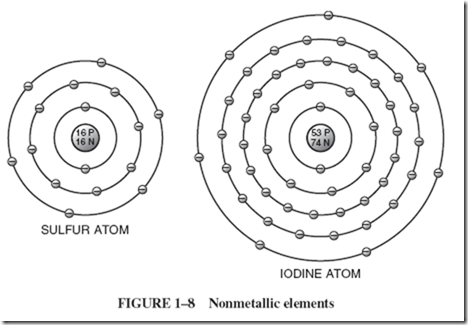
In Section 1–3 we discussed the well-known phenomenon of accumulating electrical charges on insulators, such as glass or rubber, by rubbing these substances intensely. The frictional heat causes the surface atoms to give up their valence electrons, giving rise to accumulated, nonmoving charges known as static electricity. Chapter 3 is devoted to a more detailed description of this phenomenon. |
|
2–3 ELECTRICITY FROM MAGNETISM |
|
We have already mentioned that electrical generators operate on the principle of magnetism. Magnetism plays such an important part in the future study of electricity and electronics that we will devote Chapters 15 and 16 to this subject. |
|
For our present discussion it will suffice to know of the invisible force field that exists between the north pole and the south pole of a magnet. |
|
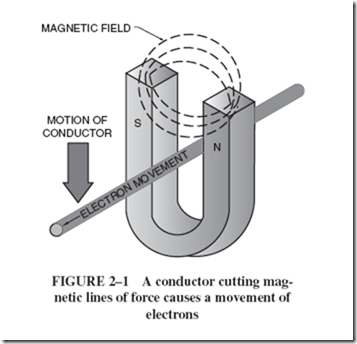
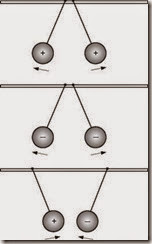
Figure 2–1 illustrates how the force of this magnetic field can be used to push the free electrons of a conductor as it is being moved within a magnetic field. This principle of inducing a current flow in a wire when it is moved within a magnetic field is known as electromagnetic induction. It is the basis of every generator, however small or large. |
|
2–4 ELECTRICITY FROM CHEMICAL ENERGY |
|
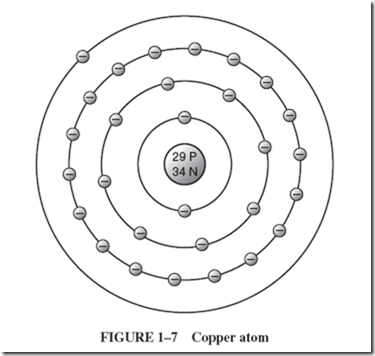
When schoolchildren experiment with basic electrical principles, they sometimes make a battery from a grapefruit or lemon. They insert two dissimilar metals into the fruit and find a small but demonstrable voltage between the two metal strips known as electrodes. The acetic acid of the fruit juice is the electrolyte, which interacts with the metals, causing a transfer of electrons. Thus, one electrode will accumulate a great surplus of electrons, making it negative with respect to the other electrode, which is considered to be positive because it suffers a shortage of electrons. |
|
The word cell refers to such a basic arrangement of a chemical substance, the electrolyte, interacting with two dissimilar electrodes. A battery is an arrangement of multiple cells. |
|
By this definition, the ordinary flashlight battery should really be called a cell, or, more commonly, a dry cell. When new, such common dry cells yield 1.5 volts. By contrast, a 12-volt car battery is a true battery, comprised of six wet cells, each providing 2 volts. Understand that all cells are basically wet. Dry cells, however, contain electrolyte as a moist paste. With a paste electrolyte and sealed construction, a dry cell provides the advantage of being functional in any position. |
|
Some cells are classified as primary cells because their chemical materials are used up as electrical energy is being produced. Primary cells are discarded after they are run down. |
|
By contrast, secondary cells can be recharged, because the chemical reaction within the cell is reversible. Car batteries, for example, are composed of secondary cells. |
|
You should remember that cells and batteries have a fixed polarity, which gives rise to a unidirectional current flow; in other words, cells and batteries always deliver direct current. There is no such thing as an AC battery. |
|
Cells and batteries are explained in greater detail in Chapter 14 of this book. |
|
2–5 ELECTRICITY FROM LIGHT |
|
Most of the life-sustaining energy encountered on our planet is derived from the sun in the form of heat and light energy. |
|
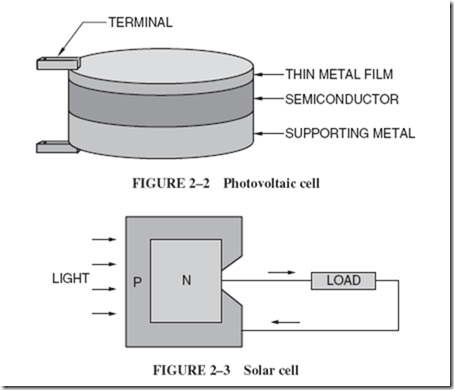
Light energyaccording to one theory of physics, is transmitted by small particles known as photons. When light strikes the surface of certain materials, called semiconductors, the photons jar electrons loose from their low-energy state, and the semi conductor accumulates opposite charges similar to those of a battery. Such a device, shown in Figure 2–2, is known as a photovoltaic cell. |
|
The solar cell is an improved type of photovoltaic cell. Specially treated silicon wafers, known as P-type silicon and N-type silicon, are fused together to form a junction. When photons strike such a device, opposite charges accumulate along the junction, producing electrical energy, Figure 2–3. |
|
The photovoltaic devices described in this section must not be confused with photoconductive devices. Photoconductive devices do not produce electricity but change their internal resistance when struck by light. One of the most common photoconductive devices is the cad cell. It exhibits a resistance of approximately 50 ohms in direct sunlight and several hundred thousand ohms when in darkness. |
|
The open-circuit voltage of a solar cell is about 0.5 volt. Voltage and efficiency (up to 25%) are fairly independent of the amount of illumination, but more light increases the current. |
|
Solar batteries are used to energize electronic equipment in artificial satellites. The communication satellite Telstar is powered by 3,600 solar cells. Solar batteries are also used for maintaining a charge on storage batteries used for rural telephone systems. |
|
2–6 ELECTRICITY FROM HEAT |
|
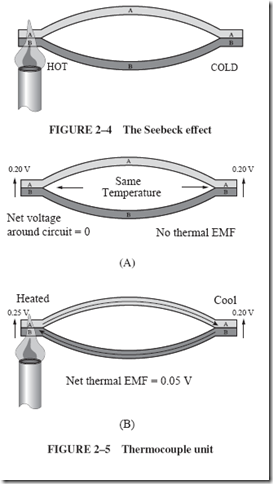
Thermoelectric effects utilizing temperature differentials to generate an electrical voltage have been known for a long time. As early as 1822, a German scientist named Seebeck showed that a circuit, such as in Figure 2–4, produces a steady current as long as the two junctions are at different temperatures. |
|
The letters A and B in the drawing represent two different metals or, possibly, different semiconductors. This direct production of an electromotive force (emf) from heat is sometimes referred to as the Seebeck effect. |
|
The explanation for the production of thermal voltage is found by a study of electron energies in conductors. When any two dissimilar metals, such as copper and iron, are in contact with each other, there is a tendency for a few electrons to drift out of one material and into the other. This slight accumulation of electrons causes a so-called contact potential difference between the materials. |
|
It is a small voltage, difficult to measure and usually noticed only as a nuisance in delicate measurements. As shown in Figure 2–5, application of heat changes the contact |
|
voltage at the heated junction, and the difference in the contact voltage at the two junctions is the useful thermal emf. |
|
Thermocouple is the name given to devices that produce a small emf when the junction of two dissimilar metals is being heated. The voltage output is a direct function of the amount of heat applied and can be used to measure temperature, especially temperatures beyond the range of liquid-containing glass thermometers. Thermocouples produce very small voltages rated in millivolts only. (Remember, 1 millivolt = 0.001 volt.) |
|
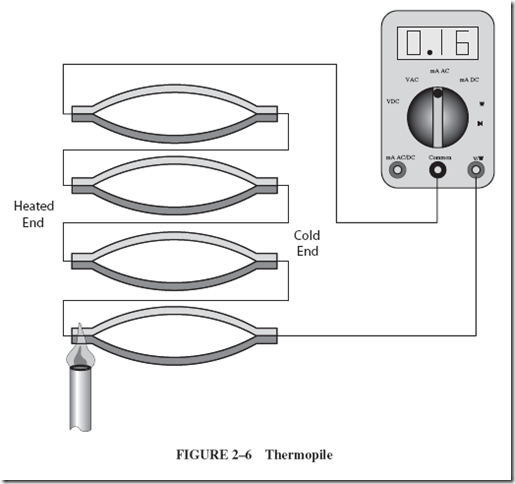
Increased voltage output can be achieved when several thermocouples are placed in series, as in Figure 2–6. Such an arrangement is called a thermopile. |
|
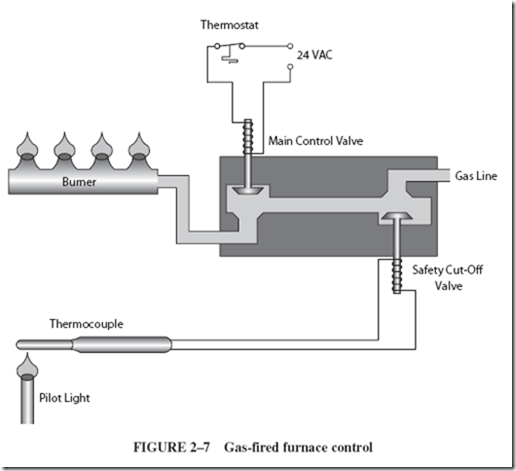
Gas-fired heating equipment is commonly controlled by an arrangement such as shown in Figure 2–7. The thermostat is a switch that closes when the room cools and turns on the gas supply to the furnace. The incoming gas is ignited by a pilot light. The pilot also heats a thermocouple, producing current so that the relay coil can hold switch S closed. If the pilot flame fails, switch S opens so that the main gas valve cannot be opened by the thermostat. Thus, the thermocouple acts as a safety device, preventing an accumulation of unburned gas in the furnace area. |
|
2–7 ELECTRICITY FROM MECHANICAL PRESSURE: PIEZOELECTRICITY |
|
The term piezo (pronounced pee-ay´-zo) means pressure. Some materials develop opposite electrical charges on opposite sides when they are compressed (or twisted, bent, or stretched). The most common application of this effect is in crystal microphones and crystal pickups for record players. Rochelle salt (sodium potassium tartrate) crystals are twisted back and forth by the sideways vibration of the phonograph stylus, producing alternating voltage on opposite sides of the crystals. That voltage, containing all the elements of speech or music, is used as the input signal voltage for an amplifier. |
|
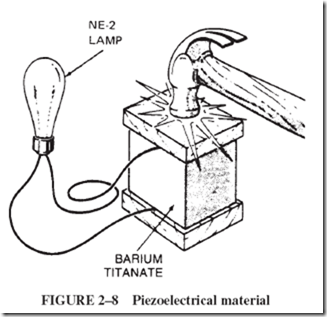
Some ceramic materials, such as barium titanate, show this piezoelectric property and can be used in record player pickups. A piece of barium titanate, when tapped with a heavy object, Figure 2–8, produces enough voltage to flash a small neon lamp of the type NE-2 (0.04 W). Wires from the lamp contact each end of the piezoelectric material. As shown in the picture, a scrap of hard plastic is placed as a cushion between the hammer and the brittle barium titanate. |
|
2–8 THE EFFECTS OF ELECTRICITY |
|
Up to this point we have been concerned with the conversion of various energy into electrical energy. It should come as no surprise that this process is revers ible at the consumer’s end. After all, the production of light and heat from electrical power is so common in our daily lives that we need hardly mention it. But what of the other forms of energy that we discussed earlier? Some of their uses may not be obvious, so let us explain. |
|
Chemical Reaction from Electricity |
|
Certain industrial processes call for chemical reactions that can be initiated by an electrical current. This process, known as electrolysis, uses the electrical current to produce desirable chemical changes or variations in the properties of certain substances. An obvious application is found in the generation of voltages from chemical batteries, as explained in Section 2–4 and in Chapter 14. Electrolysis also finds application in the electroplating of metals, the production of chemicals, the refining of copper, and the extraction of aluminum and magnesium from their ores. |
|
Mechanical Pressure from Electricity: The Piezoelectric Effect |
|
We have seen, in Section 2–7, that the slight bending of a quartz crystal causes an alternating voltage to appear on its faces. This effect can also be reversed by applying opposite charges (voltage) to the faces of a slice of quartz so that the quartz crystals bend slightly. If the charges are reversed repeatedly (by an application of AC) at a frequency that is close to the natural mechanical vibration frequency of the crystal, the crystal begins to bend and vibrate rapidly back and forth. This mechanical oscillation, in turn, sustains the continued production of alternating charges on the faces of the crystal. Since a given crystal will oscillate at only one frequency, |
|
the quartz crystal is used to control the frequency of rapidly alternating voltages in radio transmitters. In other words, vibrating crystals are used in electronic circuits where high-frequency AC is being generated and stabilized. Crystals have found wide application in two-way radio communication sets. |
|
Vibrating crystals are also used in small earphones, where the crystal vibrates a diaphragm, causing audible sound waves. Similarly, vibrating crystals have been used successfully in the production of small microphones used in cell phones. |
|
Vibrating crystals can also be used in the production of ultrasonic vibrations (approximately 50,000 cycles per second), which are used for medical purposes or in ultrasonic cleaners. |
|
Industry uses piezo materials as transducers for pressure indicators or indicators of mechanical vibration of machine parts. |
|
Magnetism from Electricity |
|
Electricity and magnetism are two closely related phenomena. Whenever an electrical current flows, magnetic forces are being created. This effect is known as electro magnetism. |
|
Electromagnetism finds countless applications in a multitude of electrical and electronic devices and appliances. For example, every electrical machine or appliance producing motion is bound to have electromagnetic forces at work. Just think of all the household appliances driven by an electric motor. All electric motors operate on electromagnetism. |
|
Without electromagnetism there would be no radio and television as we know them because the sound from the loudspeaker and the picture on your TV screen are produced electromagnetically. |
|
These are just a few examples highlighting the importance of magnetism produced by electricity. The subject of electromagnetism is covered more extensively in Chapters 15 and 16. |
|
SUMMARY |
|
• Electricity is produced by conversion from other energy sources, for example, friction, heat, light, magnetism, pressure, and chemical activity. |
|
• Friction between certain insulating substances gives rise to static electricity. |
|
• Electrical generators operate on electromagnetic principles. |
|
• Photovoltaic devices convert light energy directly into electrical energy. |
|
• Piezoelectricity (pressure emf) is a voltage developed by mechanically distorting certain crystals and ceramics. This emf is used in pickups and control elements, not as a power producer. |
|
• Heat applied to the junction of two metals will displace electrons from one metal to the other. This emf is used in thermocouples for high temperature measurements. |
|
• Batteries produce DC electricity from chemical energy. |
|
• Cells and batteries are classified as being either primary or secondary. |
|
• When electricity is used, its energy reverts back to any of the original source energies. In other words, the effects of electricity can be either heat, light, magnetism, pressure, or chemical activity. |
|
Achievement Review |
|
1. Name five methods (energy conversions) to generate electricity. Give an example of each. |
|
2. What is meant by the term electromagnetic induction? |
|
3. Explain the difference between the words photovoltaic and photoconductive. |
|
4. Name at least two applications for: • Thermocouples • Piezoelectricity • Electrolysis • Electromagnetism |