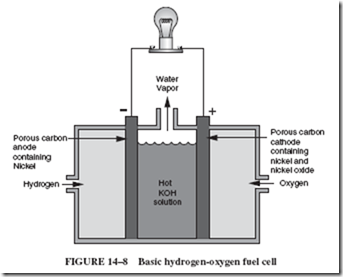
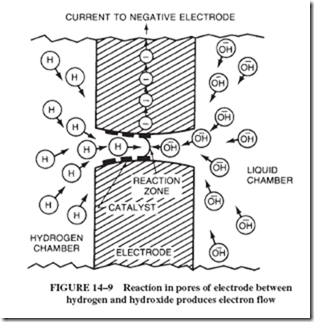
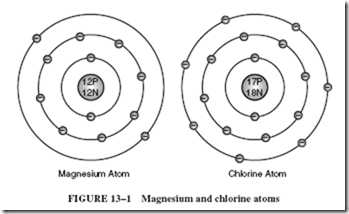
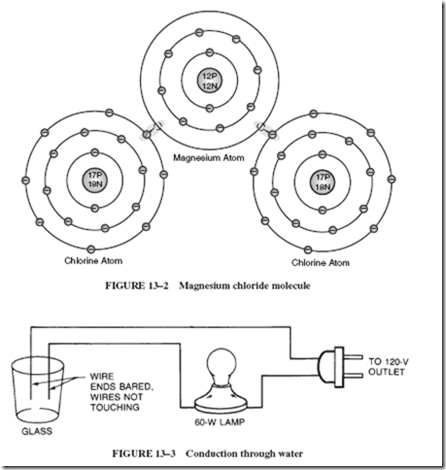
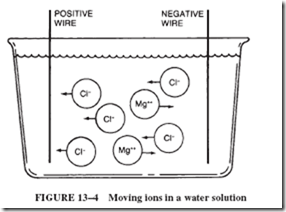
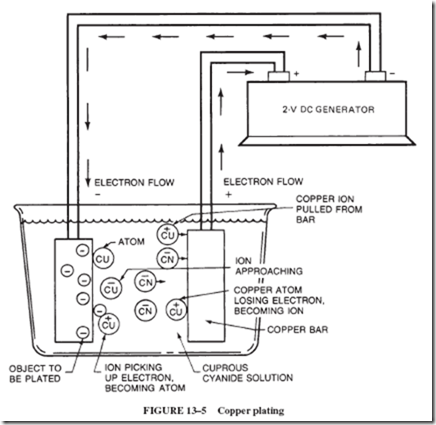
14–6 MAINTENANCE-FREE BATTERIES
The previous discussions, “Battery Testing” and “Battery Care,” become irrelevant when dealing with maintenance-free batteries, which have become popular in recent years. This kind of battery, although based on the same chemical process as the lead-acid cell, offers distinct ad- vantages over the traditional lead-acid battery. The sealed design prevents leakage of electrolyte and acid fumes, helping to eliminate corrosion problems. Since there is no loss of water, the electrolyte never needs replenishment. In addition, a well-sealed battery can be installed in any position and still function efficiently without danger of electrolyte spillage.
These features are possible because of the unique construction of the cells. The thin, low-volume separators that are normally placed between the electrodes of ordinary lead- acid batteries are replaced with thick, highly absorbent, feltlike, glass fiber mats. These separators absorb all the electrolyte, leaving no excess liquid to slosh around. Such cells are said to operate in a starved electrolyte mode, because there is only enough electrolyte to maintain the rated capacity of the cell. The cell is then sealed.
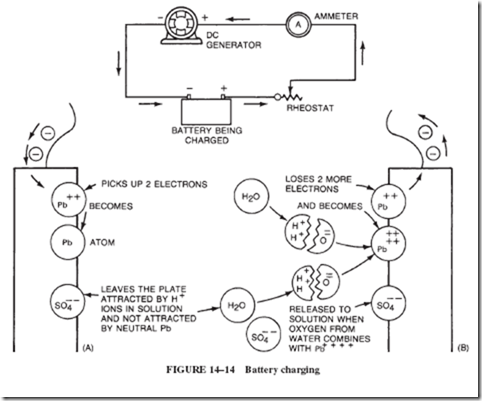
One may ask what happens to the hydrogen and oxygen gases that are generated during the charging of a lead-acid battery and become trapped inside the gas-tight container. The answer is that the design of the sealed battery allows the gases to recombine internally, with the result that water, rather than being lost, is electrochemically recycled. This is achieved by allowing the oxygen, which is generated at the positive plate, to migrate toward the negative plate, where it combines with the pure lead grids used in this construction. Likewise, the hydrogen will react with the lead dioxide of the positive plate, although at a somewhat lower rate. This recombination of gases is possible because there is just enough electrolyte to cover the plates and the glass mat separators, enabling the gas transfer between the plates. To encourage the recombination of the gases, the battery operates at a slight internal pressure — between 30 and 50 pounds per square inch. This condition is maintained by a pressure relief valve that opens only when the recommended charging rates are greatly exceeded.
It is important to note that not all “maintenance-free” batteries are perfectly sealed; some are vented to allow the escape of gases in the event of an overcharge. Since these gases are derived from the decomposition of the water in the electrolyte, such batteries can lose water, which cannot be replaced, thereby shortening their life span.
Nickel-Cadmium and Nickel-Iron (Edison) Storage Batteries
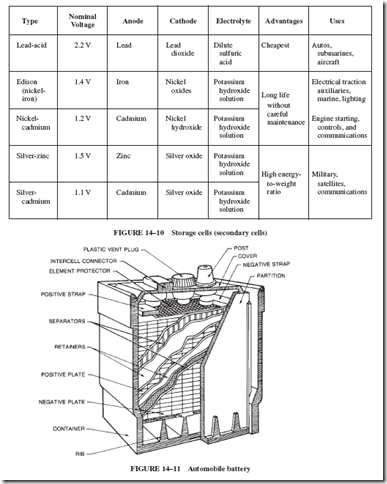
These truly long-life batteries are unaffected by the mechanical or electrical misuse that spoils a lead battery. But they cost several times as much as lead batteries of similar size. Nickel and cadmium are much more costly than lead. In addition, expensive manufacturing processes are required for these batteries.
The nickel-iron (Edison) cell was originally developed in 1899 for electric motor-driven vehicles. Research was aimed at producing a battery having low weight and volume for a given ampere-hour rating. One that could withstand repeated cycling (frequent charging and complete discharging) was especially desired.
The Edison cell is structurally stronger and lighter in weight than lead cells of the same current rating. The negative plates consist of a nickeled steel grid containing powdered iron, with some FeO and Fe(OH)2. The iron is the source of the electrons, which are
attracted through the external circuit toward nickel ions, Ni11 and Ni111, on the positive
plate. The positive plates are nickel tubes containing a mixture of nickel oxides and hydroxides, with flakes of pure nickel for increased conductivity. The electrolyte solution is 21% KOH (potassium hydroxide, caustic potash), which is chemically a base rather than an acid. The Edison cell is therefore called an alkaline cell.
The Edison cell has two disadvantages: the initial cost is high, and the maximum current is limited by high resistance, especially when the cell is cold. It is not suitable for starting gasoline or diesel engines, because its internal resistance puts a limit on its current output. These disadvantages limit its use. However, the Edison cell has an advantage that makes it useful for specific purposes. It is not damaged by remaining in a discharged condition; there- fore, it is useful in some portable lighting equipment and in a few marine installations, where it neither receives nor needs the attention that lead cells require. It is also appropriate for running traction equipment, such as mine locomotives and forklift trucks.
The nickel-cadmium (ni-cad or Ni-Cd) battery (Junger & Berg, Sweden, 1898) was developed not for frequent cycling but rather for general purposes. It enables the user to draw as many amperes as possible from a battery of given ampere-hour rating, without an excessive decline in voltage.
Both sets of plates are mechanically alike. The active materials are held in finely perforated, thin, flat steel pockets that are locked into a steel frame. The active material put in the positive plate is nickel hydroxide mixed with graphite to improve conductivity. Cadmium oxide is put into the negative plates. The electrolyte is potassium hydroxide (KOH) of about
specific gravity. When the cell is charged, the electrons forced onto the negative plate combine with Cd++ ions of the cadmium oxide (CdO), converting them to uncharged cadmium metal atoms. On the positive plate, electrons are removed from Ni++ ions of Ni(OH)2, converting them into more strongly positively charged Ni+++ ions. (The compound changes to Ni(OH)3.) Therefore, the active materials in the charged cell are

The H2O that is formed stays tied up in combination with the Ni(OH)2 in solid form on the plate and does not dilute the electrolyte; therefore, the density of the electrolyte remains constant, and a hydrometer does not indicate the amount of charge.
The nickel-cadmium battery is similar in its chemical composition to the Edison cell. Both contain alkaline electrolytes. Its electrical characteristics are similar to those of the lead cell; therefore, its applications are competitive with those of the lead cell. Like the Edison cell, the nickel-cadmium battery is chiefly used in industrial service. Its ruggedness, long life, and low-maintenance cost outweigh the high original cost. (Nickel-cadmium batteries cost nearly twice as much as do lead acid batteries of comparable energy ratings.)
Nickel-cadmium cells are marketed in the popular sizes of zinc-carbon cells (sizes AA, A, C, and D) for replacement in toys and consumer appliances. The buyer should con- sider the following potential disadvantages:
1. For any given number of cells, the voltage output is only 80% that of dry cells.
2. Unused nickel-cadmium cells tend to lose a significant amount of their emf when standing idle for several days. This effect is aggravated by higher temperatures.
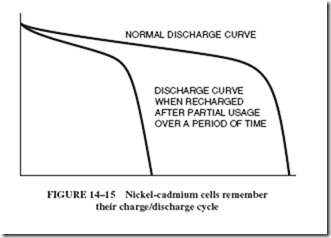
3. Nickel-cadmium batteries tend to remember repeated demands for low output and sometimes persist in delivering such low levels, even though they are designed to meet the needs of increased output.
4. Nickel-cadmium cells do exhibit one negative characteristic. They remember their charge/discharge cycles. If they are used at only low currents, are permitted to dis- charge through only part of a cycle, and then are recharged, over a period of time they will develop a characteristic curve to match that cycle; see Figure 14–15.
On the positive side, it should be mentioned that nickel-cadmium batteries are good for nearly 2,000 recharge cycles and can be left on a trickle charge for indefinite periods of time.
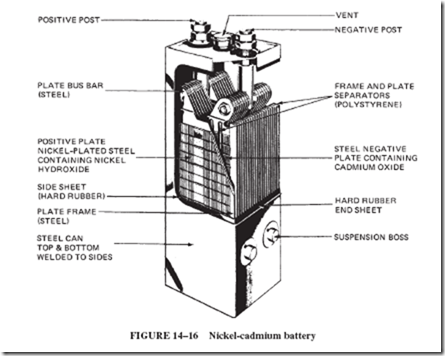
Commercially, the nickel-cadmium battery is used in railroad signals systems; fire alarm systems; relay and switchgear operation; missile controls; aircraft engines; and diesel engines in locomotives, buses, and oil well pumps. Figure 14–16 shows a large, industrial- type cell. Nickel-cadmium cells in smaller, sealed, cylindrical forms, with no problems of gas or spillage, are used in a great variety of communication equipment and portable appliances.
Nickel-Metal Hydride Cells
Nickel-metal hydride cells (Ni-MH) are similar to nickel-cadmium cells in many respects. Both exhibit a voltage of 1.2 volts per cell and both have very similar charge and discharge curves. Nickel-metal hydride cells exhibit some improved characteristics, however. They have about a 40% higher energy density, and redundant memory accumulation is not as great a problem. Nickel-metal hydride cells are also more environmentally friendly. The posi- tive electrode is made of nickel oxyhydroxide (Ni-OH) and the negative electrode is made of metal hydride. The electrolyte is an aqueous (watery) potassium hydroxide solution. Nickel- metal hydride cells are replacing nickel-cadmium cells in many applications.
Lithium-Ion Cells
Lithium-ion cells are very popular for portable equipment such as notebook computers, video cameras, cell phones, and many others. Lithium-ion cells can be recharged and offer a very high energy density for their size and weight. They exhibit a voltage of 3.6 volts per cell, which is the same voltage that can be obtained by connecting three nickel- cadmium or three nickel-metal hydride cells in series. Lithium-ion cells also exhibit a weight-energy density that is about three times greater than nickel-cadmium cells. Under proper charging conditions, these cells can be recharged about 500 times. They also exhibit
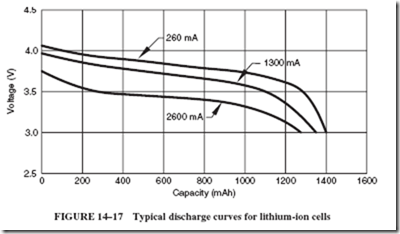
a rather flat discharge curve, as shown in Figure 14–17, which makes them an ideal choice for electronic devices that require a constant voltage. Unlike nickel-cadmium cells and to some extent nickel-metal hydride cells, lithium-ion cells do not have the problem of memory accumulation. They can be recharged to their full capacity each time they are charged.
Lithium-ion cells can be safely recharged because they do not contain metallic lithium. The positive electrode (or cathode) is made of lithium metallic oxide and the negative electrode (or anode) is made of carbon. These cells work by transferring lithium ions between the cathode and the anode during discharging and charging. Although lithium-ion cells are safe to recharge they do require a special chargers that generally produce a constant voltage and constant current. Too much current or voltage during charging can cause early deterioration of the cell.
14–7 MISCELLANEOUS ASPECTS OF BATTERIES
Internal Resistance (R1) of Cells
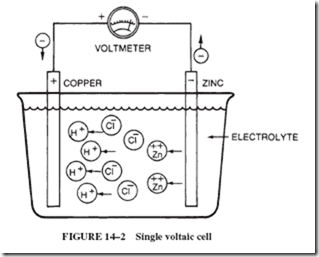
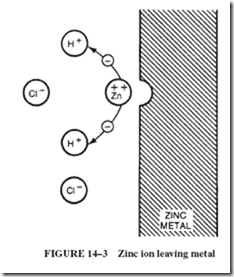
Cells themselves have an internal resistance that enters into a circuit calculation. When electrons flow from the negative plate to the positive plate, there is a movement of ions (cur- rent) in the electrolyte in the cell. This movement in the cell, like any current, does not occur with perfect ease; there is some resistance in the internal material of the cell.
This internal resistance of the cell (or battery) is generally shown in series with the load; see Figure 14–18. This means that every time current flows in the circuit, a voltage drop (IR drop) will occur across the internal resistance. This voltage drop subtracts from the battery’s emf and is lost to the load.
Let us assume that a 12-volt car battery has an internal resistance of 0.2 ohms and is connected to a load that draws 5 amperes. The internal voltage drop of the battery will be = I x R = 5 x 0.2 = 1 volt. In other words, the load receives only 11 volts, as shown in Figure 14–19A.
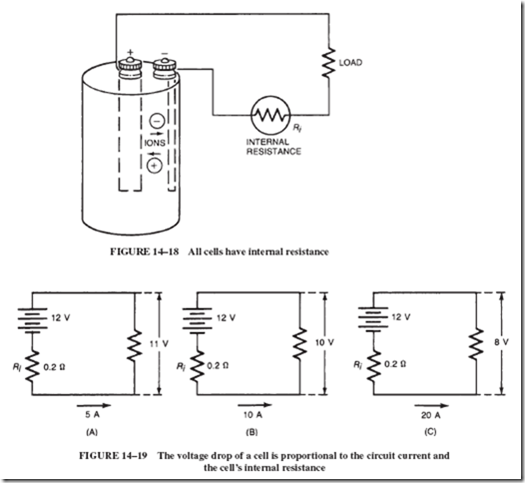
If the current is doubled to 10 amperes, the corresponding voltage drop will be 2 volts, and consequently the load “sees” only 10 volts; see Figure 14–19B. Likewise, if the load current is increased to 20 amperes, the load voltage would be only 8 volts; see Figure 14–19C.
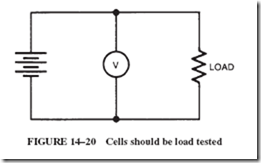
The internal resistance of a cell increases with age, which in turn diminishes the voltage delivered to the load. Such a run-down battery may still yield a good reading when checked with a voltmeter. This happens because high-quality voltmeters have a very high resistance and draw virtually no current from the source. In other words, checking a battery’s condition with a voltmeter can be meaningless unless the test is performed when the battery is loaded down; see Figure 14–20.
Maximum Current from a Cell
A cell produces its greatest current, uselessly, when it is short-circuited. Assume we connect a wire of practically 0 ohms resistance across the terminals of a 1.5-volt, 0.035-ohm dry cell. The amount of current is limited only by the internal resistance of the cell: I = 1.5 ÷ 0.035 = 42.8 amperes. The terminal voltage is now 0, because all of the cell emf is used inside the cell. If this condition exists for more than a few seconds, the cell overheats, gases form in it, the electrolyte starts boiling out the top of the cell, and the cell is destroyed.
If a dry cell is used to produce a moderate current for 10 or 15 minutes, there can be a noticeable drop in terminal voltage and current by the end of this time. This drop is caused by the temporary increase in internal resistance due to the formation of a very small amount of hydrogen around the positive plate. When the cell is allowed to stand on open-circuit, this hydrogen is reconverted to H2O, and the cell is restored to its original low internal resistance.
Dry cells generally fail because they really become internally dry. An unused dry cell on a warm shelf for 2 years may lose its moisture by evaporation, despite the manufacturer’s attempt to seal the top. A cell in use loses its moisture when a hole is finally dissolved in the zinc case. Drying out causes a great increase in internal resistance. A cell with 1 or 2 ohms internal resistance is of no value. It may produce enough current to make a voltmeter read 1.5 volts but not enough current to light a flashlight bulb brightly.
Cells Connected in Series
The voltage output from series-connected power sources can be additive or sub- tractive, depending on the polarity of the power sources. This was fully explained in Section 10–11; review that material, if necessary.
Cells Connected in Parallel
When multiple power sources are connected in parallel, there is no increase in emf. Why, then, would anybody want to connect batteries in parallel?
1. The current capacity of such an arrangement is increased. In other words, three cells can deliver three times as much current as one cell can.
2. The life span of such a parallel arrangement is increased. This is an advantage in that it is more convenient to replace a group of three cells every 30 days than to replace one cell every 10 days.
3. The internal resistance of the parallel group is less than that of a single cell; there- fore, the terminal voltage will be closer to the battery emf.
When batteries or other power sources are connected in parallel, it is important to observe the proper polarity (positive to positive and negative to negative) and to ensure that all batteries have equal voltages.
Cells Connected Series-Parallel
If the current and voltage requirements of a load are higher than those of a single cell, a series-parallel arrangement of multiple cells is in order. An example will make this clear.
EXAMPLE 14–1
Given: A load rated at 6 volts and 2.4 amperes; available cells are rated at 1.5 volts and 800 milliamperes.
Find: A suitable arrangement of series-parallel-connected cells to satisfy the load requirement.
Solution
1. Connect four 1.5-volt cells in series to satisfy the voltage requirement of 6 volts.
Such an arrangement of cells, known as a series-connected bank, illustrated in Figure 14–21A, is capable of delivering no more current than one single cell; in this case, 0.8 ampere.
2. Connect additional banks of series-connected cells to increase the current capacity of the bank. In this case, 2.4 divided by 0.8 5 three banks, as shown in Figure 14–21B.
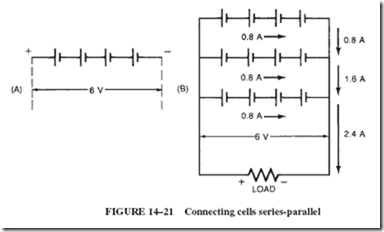
Note: The current capacity of a battery is equal to that of one cell multiplied by the number of parallel banks.
Advantages and Disadvantages of Batteries
Advantages of batteries are that they are portable, reliable, and self-contained. Sub- marines, automobiles, aircraft, and flashlights for small boys are four examples of devices that need to be independent of power line operation. Power line service can be interrupted by storms. In such situations, batteries can provide emergency lighting and communication service.
The great disadvantage of batteries is that the energy they produce is expensive, so batteries are used only where the convenience outweighs the cost. For example, how much energy is there in an automobile battery? A 6-volt car battery can produce 6 amperes for 20 hours (120 ampere-hours). Watt-hours of energy is volts × amperes × hours = 6 × 6 × 20 = 720 watt-hours, which is about 0.75 kilowatt-hour. Power corporations charge much less for 1 kilowatt-hour of energy. An ordinary flashlight cell (size D) contains a little over 0.5 ounce of zinc. According to the chemistry book, this amount of zinc is 1.5 × 1023 atoms. Each atom supplies 2 electrons, so if all of the zinc is dissolved usefully, 3 × 1023 electrons are produced. One coulomb is 6 3 1018 electrons; 3 × 1023 electrons is equal to 50,000 coulombs.
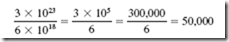
It takes 3,600 coulombs to equal an ampere-hour, so the flashlight cell, even if 100% efficient, can produce only 14 ampere-hours. Fourteen ampere-hours at 1.5 volts equals 21 watt-hours. Therefore, 50 flashlight cells would be needed to produce 1 kilowatt-hour of energy.
Similar calculations can be made to show even more drastic examples of the cost of energy provided by cells. For example, if one considers the price paid for those tiny silver- oxide cells used to power electronic watches and hearing aids, the cost of energy obtained from such cells might amount to more than $3,000 per kilowatt-hour.
Safety Precautions with Batteries
1. The hydrogen and oxygen released by a battery being charged form an explosive gas mixture that can be ignited by sparks or open flames.
2. If acid needs to be diluted with distilled water (for use as an electrolyte), never pour water into acid. Such action may cause splattering and serious acid burns. Instead, slowly pour the acid into the water.
3. When handling acids, wear safety goggles, rubber gloves, rubber aprons, or similar protection garb.
SUMMARY
• Batteries consist of cells that deliver an emf from chemical energy.
• Primary cells cannot be recharged when run down.
• Secondary cells are rechargeable.
• The emf obtainable from a cell depends on its chemical makeup.
• The current rating, or ampere-hour capacity, of a cell depends on the size of its electrodes.
• Cells have an internal resistance that causes them to drop some of their emf. This IR drop is the difference between their open-circuit and closed-circuit voltage.
• Voltage readings from batteries should be taken under loaded conditions.
• Cells connected in series can deliver more voltage.
• Cells connected in parallel can deliver more ampere-hours.
Achievement Review
1. Define the words primary cell and secondary cell.
2. What is meant by the word electrolyte?
3. Explain the term specific gravity.
4. What kind of instrument is used for testing the specific gravity?
5. Someone reports the specific gravity of a battery to be 1,100. What does that say about the battery?
6. What is meant by the term ampere-hour?
7. Name two common types of secondary cells.
8. A battery is generally considered to be a source. Can it ever be regarded as a
load? Explain your answer.
9. Why do most battery chargers contain a rectifier?
10. Is it advisable to store batteries in a discharged condition? Why or why not?
11. What kind of substance is recommended to clean and neutralize corrosion on the terminals of a battery?
12. Why does a discharged battery freeze more easily than a fully charged battery?
13. Why should lighted matches never be used for illumination when inspecting the electrolyte of a battery?
14. Is the voltage output of a battery dependent on
a. The size of the plates?
b. The number of plates? Explain.
15. Draw four cells connected
a. For highest voltage output
b. For maximum life expectancy and high current output
16. Two 24-volt batteries in series are being charged by a 60-volt generator. Each battery has 0.02-ohm internal resistance. Calculate how much additional resistance is needed in the circuit to limit the current to 6 amperes.
17. Make a drawing to show how seven dry cells (1.5 V each) can be connected to yield 6 volts.
18. Now show seven wet cells (2 V each) connected to yield 10 volts.
19. In the following schematics, you see different circuit arrangements of batteries composed of 1.5-volt dry cells.
a. Check each one of the circuits to see if it is properly or incorrectly connected.
b. Estimate the voltage output of each combination from part a.
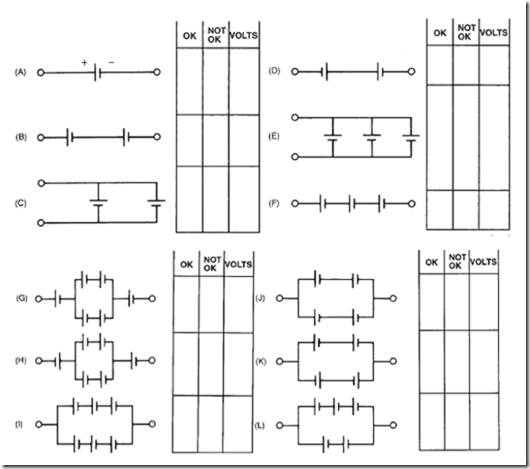
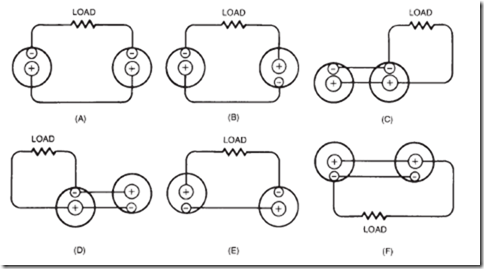
20. The sketches below represent various possible ways of connecting two dry cells and a lamp (as viewed from the top). The 1 and – are the terminals of the cells; the resistance represents the lamp. For each: State the voltage at the lamp (0, 1.5, or 3 V). Classify the circuits as good or poor.
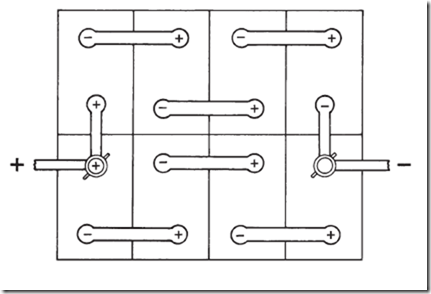
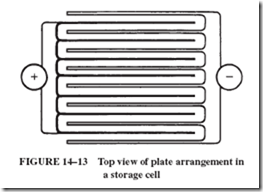
21. Eight lead storage cells are arranged as in the drawing below (top view). What is the emf of this battery?