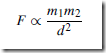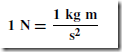Force, Mass and Acceleration
Introduction
When an object is pushed or pulled, a force is applied to the object. This force is measured in newtons (N). The effects of pushing or pulling an object are:
(i) to cause a change in the motion of the object, and
(ii) to cause a change in the shape of the object.
If a change occurs in the motion of the object, that is, its velocity changes from u to v, then the object accelerates. Thus, it follows that acceleration results from a force being applied to an object. If a force is applied to an object and it does not move, then the object changes shape, that is, deformation of the object takes place. Usually the change in shape is so small that it cannot be detected by just watching the object. However, when very sensitive measuring instruments are used, very small changes in dimensions can be detected.
A force of attraction exists between all objects. The factors governing the size of this force F are the masses of the objects and the distances between their centres:
Thus, if a person is taken as one object and the earth as a second object, a force of attraction exists between the person and the earth. This force is called the gravitational force and is the force that gives a person a certain weight when standing on the earth’s surface. It is also this force that gives freely falling objects a constant acceleration in the absence of other forces.
Newton’s Laws of Motion
To make a stationary object move or to change the direction in which the object is moving requires a force to be applied externally to the object. This concept is known as Newton’s first law of motion and may be stated as:
An object remains in a state of rest, or continues in a state of uniform motion in a straight line, unless it is acted on by an externally applied force
Since a force is necessary to produce a change of motion, an object must have some resistance to a change in its motion. The force necessary to give a stationary pram a given acceleration is far less than the force necessary to give a stationary car the same acceleration. The resistance to a change in motion is called the inertia of an object and the amount of inertia depends on the mass of the object. Since a car has a much larger mass than a pram, the inertia of a car is much larger than that of a pram.
Newton’s second law of motion may be stated as:
The acceleration of an object acted upon by an external force is pro- portional to the force and is in the same direction as the force Thus, force ˛ acceleration, or force D a constant ð acceleration, this constant of proportionality being the mass of the object, i.e.
force = mass × acceleration
The unit of force is the newton (N) and is defined in terms of mass and acceleration. One newton is the force required to give a mass of 1 kilogram an acceleration of 1 metre per second squared. Thus
F = ma
where F is the force in newtons (N), m is the mass in kilograms (kg) and a is the acceleration in metres per second squared (m/s2), i.e.
It follows that 1 m/s2 D 1 N/kg. Hence a gravitational acceleration of 9.8 m/s2 is the same as a gravitational field of 9.8 N/kg
Newton’s third law of motion may be stated as:
For every force, there is an equal and opposite reacting force
Thus, an object on, say, a table, exerts a downward force on the table and the table exerts an equal upward force on the object, known as a reaction force or just a reaction.
For example, to calculate the force needed to accelerate a boat of mass 20 tonne uniformly from rest to a speed of 21.6 km/h in 10 minutes:
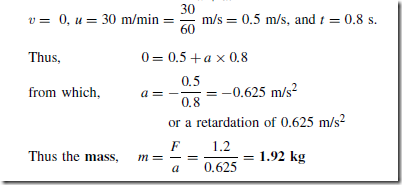
In another example, if the moving head of a machine tool requires a force of 1.2 N to bring it to rest in 0.8 s from a cutting speed of 30 m/min, then from Newton’s second law, F D ma, from which, the mass of the moving head,
The law of motion v D u C at can be used to find acceleration a, where
Centripetal Acceleration
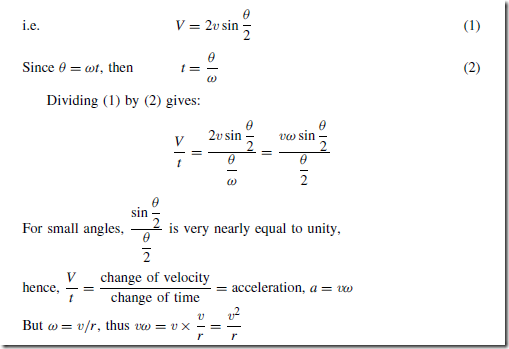
When an object moves in a circular path at constant speed, its direction of motion is continually changing and hence its velocity (which depends on both magnitude and direction) is also continually changing. Since acceleration is the (change in velocity)/(time taken) the object has an acceleration.
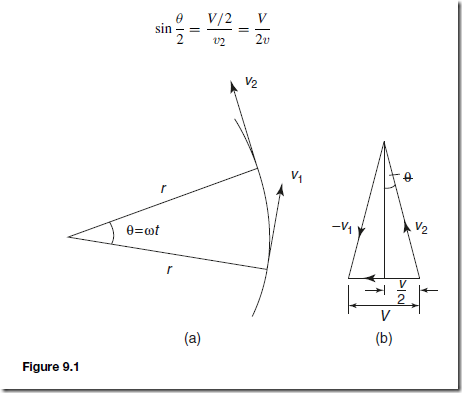
Let the object be moving with a constant angular velocity of ω and a tangential velocity of magnitude v and let the change of velocity for a small change of angle of ˛ˇD ωt) be V (see Figure 9.1(a)). Then, v2 – v1 D V.
The vector diagram is shown in Figure 9.1(b) and since the magnitudes of v1 and v2 are the same, i.e. v, the vector diagram is also an isosceles triangle.
Bisecting the angle between v2 and v1 gives:
That is, the acceleration a is ![]() and is towards the centre of the circle of motion (along V). It is called the centripetal acceleration. If the mass of the rotating object is m, then by Newton’s second law, the centripetal force is
and is towards the centre of the circle of motion (along V). It is called the centripetal acceleration. If the mass of the rotating object is m, then by Newton’s second law, the centripetal force is![]() , and its direction is towards the centre of the circle of motion.
, and its direction is towards the centre of the circle of motion.
For example, if a vehicle of mass 750 kg travels round a bend of radius