13–4 GASES AS INSULATORS
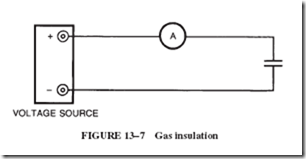
At atmospheric pressure, air and other gases are very close to being ideal insulators. When a gas is used as an insulator between two charged plates, as illustrated in Figure 13–7, the current shown by the ammeter is practically zero as long as a moderate voltage is applied. At a sufficiently high voltage, the gas suddenly becomes a conductor, and the current value is limited only by the circuit itself.
At less than atmospheric pressure, this breakdown in the insulating ability of a gas occurs at a lower voltage. However, if the gas pressure is increased, such as in com- pressed air, then the applied voltage necessary to start conduction in the gas must be increased a proportional amount. For example, a higher voltage is required to fire a spark plug under compression in an engine than to create the spark in the open air.
Sparks and Arcs
A spark is a noisy, irregular discharge; successive sparks follow separate paths. This statement refers to sparks that indicate electron paths. The sparks that are visible when
a wire is brushed across the terminals of a car battery are similar to the sparks from a grinding wheel; that is, they are tiny fragments of hot metal being sprayed in all directions.
An arc is quieter than a spark and is a continuous discharge. An arc, in air, consists of a conducting path of highly heated gas or metallic vapor. In this case, the air offers almost no resistance; therefore, an external resistance must be present in the circuit to limit the current. The potential drop across the arc itself is moderately low, in the or- der of 15 to 50 volts. An arc is accompanied by high-intensity light. Most of this light is due to gas or metallic vapor, but some light comes from the hot cathode. The cathode temperature of a carbon arc may reach 9,000°F.
13–5 GASEOUS CONDUCTION BY IONIZATION
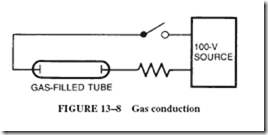
Gas can be made to conduct a current if a moderately high voltage of 50 volts or more is applied to a gas at a low pressure inside a glass tube, as shown in Figure 13–8. (Note that the wires from the voltage source are sealed inside the tube.) In air, an arc can be started by touching and then separating a pair of conducting contacts, as in a welding arc or a carbon arc.
“How does the gas conduct?” “What happens in the gas so that it becomes a conductor?” The process of ionization causes a gas to become a conductor. In this process, electrons are removed from gas atoms so they become positively charged ions. The free electrons are highly mobile. As a result, conduction in the gas is due mainly to electron flow (as in metals). Although mobile positive ions do exist (as in liquids), the nature of conduction in a gas differs greatly from conduction in either a solid or a liquid.
How Ionization Occurs
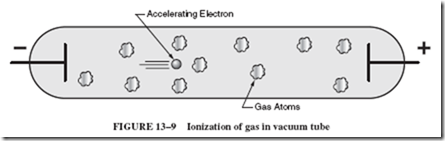
The most important process involved in ionization is electron impact. The ionization process can begin when an electron is freed from the negative wire. More likely sources of free electrons are radiation and heat (to be investigated shortly). Once an electron is set free, it gains speed in the enclosing glass tube or envelope as it is repelled by the negative
(2) wire and attracted toward the positive (1) wire; see Figure 13–9. If the electron hits an atom while it is still moving slowly, it merely bounces off the atom and again accelerates toward the positive terminal. When the electron is moving fast enough, any collision with an atom causes one or more electrons to be knocked free of the atom.
The minimum amount of energy that an electron must possess to cause ionization (removal of electrons from atoms) is called the ionization potential. The amount of
energy required depends on the kind of atom that is to provide the electrons. The ionization potential for sodium vapor is about 5 volts. For most gases, the ionization potential ranges between 10 and 25 volts (for example, mercury vapor requires 10.4 volts; neon,
volts; and helium, 24.5 volts). In other words, to ionize neon, an electron must fall through a potential difference of 21.5 volts.
The ionization process is shown in Figure 13–10. As one can see, the original colliding electron frees another electron. Now there are two free electrons that begin to accelerate toward the positive wire. These electrons collide with more atoms, and each electron frees an additional electron. Now there are four electrons. The subsequent series of collisions between electrons and atoms frees more electrons so that in a thousandth of a second, a million electrons may be released.
Let us compare this action with what happens to a heavily laden apple tree when one apple at the top starts to fall to the ground. The one apple will strike another apple, and the two apples will strike other apples, until finally, by the time the apple fall is completed, there is a bushel of apples on the ground.
Light is produced during gaseous conduction as a result of collisions that are not violent enough to free electrons. The collision of a moderately fast-moving electron with an atom can transfer some of the energy of the electron to the electrons of the atom. This transfer of energy produces an excited state in the atom. In this state, the atom has excess energy and rids itself of this energy by emitting light. The color of the emitted light is characteristic of the energy state of the atom.
A second process that produces ionization in a gas is radiation. Rays striking the earth from space may ionize a few atoms and thus provide the first electrons required
to start the collision process. After conduction begins, electron disturbances in the atoms produce visible light and high-frequency (ultraviolet) radiation. This high-frequency radiation is absorbed by other atoms, thus providing them with enough energy to free electrons.
Still another phenomenon that produces ionization is heat. A hot gas is a better conductor and begins to conduct more readily than a cold gas. Heat is the movement of atoms and molecules. At higher temperatures, collisions between atoms can become violent enough to dislodge electrons. Many of the atoms may have a higher temperature than the average and thus can cause ionizing collisions. An ordinary flame contains many ions and is therefore a poor insulator. This statement may be proved by bringing a match flame near a charged electroscope.
Another source of electrons is the collision of positive ions. Although positive ions have lost one or more electrons, they still have electrons that can be set free. Therefore, when positive ions collide, electrons are released and the ions are even more positively charged. In addition, excited gas atoms can collide with other kinds of atoms, causing these atoms to release electrons. For example, conduction can be maintained more easily in neon gas if a trace of nitrogen gas is added to the neon. Excited neon atoms will collide with the nitrogen atoms. In the process, the nitrogen atoms are given enough energy to cause them to release electrons.
Why Low-Pressure Gas Conducts Better Than High-Pressure Compressed Gas
In gas at atmospheric pressure, a free electron collides with gas atoms so frequently that it never travels long enough to gain the speed required to ionize an atom by collision; see Figure 13–11A. At lower pressures, gas atoms are farther apart; therefore, an electron has the opportunity to gain enough kinetic energy to make an ionizing collision; see Figure 13–11B. As a result, designers of gas tubes must determine the mean free path of electrons to ensure that ionizing collisions will occur. The mean free path is the average distance traveled by a particle between collisions.
Electrons and Ions Formed by Collisions
The electrons that reach the positive terminal accomplish useful conduction. How- ever, in a glass tube, many of the free electrons collect on the walls of the tube. Any positive ions that hit the walls are neutralized by the negative charges collected there.
On the average, approximately 1 atom out of every 1,000 atoms is ionized in a typical gas conduction tube. The glowing discharge in the tube is called plasma and consists of positive ions, electrons, and neutral atoms. The voltage drop in this region is not large; most of the voltage drop occurs near the electrodes. Scientists are still con- ducting experiments to define potential applications of the plasma phenomenon. For example, a number of years ago it was found that bursts of current (of a very high amperage) in hydrogen at low pressure produce plasmas that are contained by their own magnetic fields. The purpose of the experiments was to make the particles in the plasma reach speeds corresponding to temperatures of millions of degrees. It is at such temperatures that hydrogen nuclei fuse into helium. This hydrogen fusion process is the same as that occurring in the sun. Hydrogen fusion happens suddenly when an H-bomb explodes.
13–6 CONDUCTION AND IONS IN NATURE
An occasional evening celestial display in the northern skies, the Northern Lights (aurora borealis) is believed to be the result of conduction in the very thin upper part of the atmosphere. This display appears to be caused by electrons that are given off by disturbances in the sun and then travel through space until they reach the Earth’s atmosphere.
In addition to electrons, high-speed protons also reach Earth from space. These protons strike air molecules and produce intense radiation that ionizes various layers of the atmosphere. These conducting layers have the ability to reflect radio signals; these reflections are often useful.
Lightning is a high-voltage spark. At times atmospheric conditions that are not violent enough to cause lightning produce a continuous discharge that can be seen on steeple tops or the masts of ships.
In the vicinity of pointed electrodes, a strong electric field will ionize the air. The discharge that occurs is visible if other illumination does not interfere. This type of dis- charge is called a corona and occurs frequently in high-voltage equipment if precautions are not taken to prevent it. Corona effects can be a serious source of power loss in high-voltage transmission lines.
13–7 CONDUCTION IN A VACUUM
If gas is pumped from a glass tube until the pressure in the tube is reduced to approximately 0.0001 mm of mercury (760 mm of mercury 5 atmospheric pressure), the brilliant glow of the conducting gas is no longer visible. The gas molecules are so far apart that few ions are formed. In this case, conduction is due almost entirely to electron flow. The glass tubing itself may glow because of electrons striking the glass.
If the gas pressure is reduced even more in a cold cathode tube, the discharge may stop entirely. If the cathode wire is then reheated by another current, conduction through the vacuum continues by means of the electrons that escape from the hot cathode.
Over a century ago, the people who were experimenting with conduction in gases and vacuums did not know of the existence of electrons. These experimenters gave the name cathode ray to the emissions from the cathodes of their tubes. In 1869, the German scientist Johann Hittorf described the glow resulting when glass was struck by these rays. His experiments showed that the cathode rays traveled in straight lines. An English scientist, Wilhelm Crookes, found that the paths of these rays can be bent by a magnet. In addition, he found that these rays can be focused, that they heat the objects they strike, and that their speed depends on the applied voltage. In 1879, Crookes suggested that these rays might be “an ultra-gaseous state of matter.” The French investigator Jean-Baptiste Perrin showed in 1895 that the rays carried a negative charge and that positive ions were also formed.
Wilhelm Roentgen, while experimenting with cathode-ray tubes in 1895, found that when the cathode rays strike metal or glass, a new kind of radiation is produced. He deter- mined that these invisible rays passed readily through air, paper, and wood; through thin metal better than thick; through flesh better than bone; and through aluminum better than lead. The rays caused fluorescence in some minerals, affected photographic plates, and were unaffected by a magnetic field. Roentgen called these rays X rays. Within a few months of his discovery, physicians were using X rays as an aid in setting broken bones.
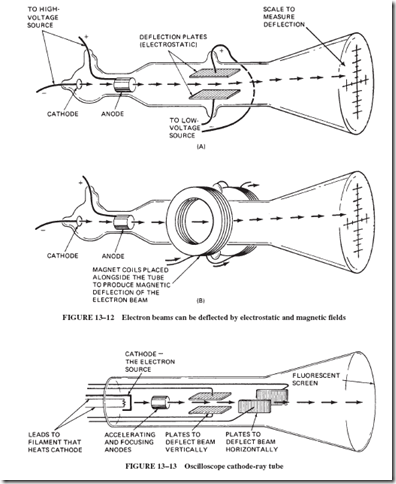
In 1897, the English scientist J. J. Thomson completed a series of experiments in which he was able to measure the ratio of the weight of the negative particle to the electrical charge; see Figure 13–12. Modern cathode-ray tubes, such as those used in oscilloscopes and TV picture tubes, use the same principles of electron ray deflection that Thomson used in his experiments.
As shown in Figure 13–13, in oscilloscopes, the electron beam is accelerated from the cathode and is deflected horizontally at a known rate. The voltage (whose trace is to be observed) is used to deflect the beam vertically at the same time. The result is a time graph of the observed voltage.
In TV picture tubes, magnetic coils are used to sweep the beam of electrons horizon- tally and vertically. At the same time, the intensity of the beam is changed to produce variations in the lightness and darkness of the picture. There continues to be some concern that harmful X rays are produced when the 20,000-volt electrons strike the face of the TV picture tube. This voltage generally is not great enough to produce the highly energetic and penetrating X rays. Any X rays that may be produced are absorbed in the glass and the few inches of air in front of the tube. A more serious source of X rays is the high-voltage rectifier tube in larger TV sets. In the past, some TV receivers radiated undesirable quantities of X rays through the bottom of the set because insufficient metal shielding was used around the high-voltage supply.
The American experimenter Thomas Alva Edison might have discovered electrons. One day in 1883, one of his assistants accidentally connected a meter to a dead-end wire sealed into one of Edison’s experimental electric lamps. When the lamp filament was heated, a small current was indicated on the meter; see Figure 13–14. The heated lamp filament was certainly emitting electrons. The free electrons in the lamp were attracted to the positive wire through the meter, thus giving rise to the current. This event was reported, but it was
not until 20 years later that J. A. Fleming constructed a two-element valve to use as a rectifier and explained the action taking place in this device. The elements are the filament, which emits electrons, and the plate, which collects the electrons when it is positive. (Valve is the British term for vacuum tube.) In later years when Edison was asked why the third wire was sealed into his lamp bulb, he is reported to have said, “I have forgotten.”
Fleming’s concept of electron conduction in vacuum tubes has been applied to hundreds of devices designed to achieve specific tasks. Research conducted to improve glass- enclosed vacuum tubes led eventually to the discovery that special solid materials have the same characteristics as the simple vacuum tubes. As a result, the field of solid-state electronics was born.
SUMMARY
• Electrical conduction in liquids is the movement of positive and negative ions. There are no free electrons as in metals.
• Positive and negative ions are formed by a type of chemical combination in which one metallic element, or group, transfers electrons to a nonmetallic element or group.
• If a chemical compound can be dissolved in water, the charged ions of the compound become freely movable in the solution.
• In electroplating processes, the solution contains ions of the plating metal. The article to be plated is connected to the negative terminal of the current source. A bar of the plating metal is connected to the positive terminal.
• Metal removed from the plating solution and deposited on the object is replaced in the solution by metal dissolved from the positive bar.
• In liquid conduction, positive ions move in one direction and negative ions move in the other direction, resulting in a permanent separation of the parts of the compound. This decomposition process is called electrolysis.
• Gas is a good insulator until sufficient voltage is applied. The sudden ionization process changes the gas to a conductor.
• Ionization of a gas is the freeing of electrons from the gas molecules. The positively charged gas particles are the positive ions. The electrons are the movable negative particles.
• Conduction in a gas consists mainly of electron movement. As electrons collide with gas atoms, a new supply of electrons is continually set free from the atoms.
• Gases at low pressure conduct more readily than gases at high pressure.
• At pressures close to a vacuum, electrons from the cathode travel in straight paths.
These electrons were called cathode rays. The discovery of electrons consisted of the measurement of their properties in cathode-ray tubes.
Achievement Review
1. How do metals differ from nonmetallic elements in the structure of their atoms?
2. What are ions?
3. Is dry salt a conductor? Why or why not?
4. Can a piece of wood be electroplated? Why or why not?
5. What happens if the wires leading to the generator in Figure 13–5 are reversed?
6. When a car battery is disconnected from the charging line by pulling the clip off the battery post, occasionally the top is blown off the battery. Why does this happen and how can it be avoided?
7. Water is regarded as an insulator. If water is an insulator, why is it that electrocution is possible by contact between a power line and wet earth?
8. Special problems in insulation had to be solved in the development of electrical control systems for high-altitude missiles. Why should any special problems exist?
9. Who discovered electrons?
10. What is the difference between cathode rays and electrons?
11. Name some useful examples of gaseous conduction.
Is it true that practically all of the useful discoveries in this field have already been made? Explain.