An Introduction to Electricity and Electronics
Almost everyone is aware of the phenomenal developments in the field of electronics technology during the recent decades. The very term electronics evokes visions of exotic and complex devices that are quickly altering our individual and collective life styles. |
|
The study of electricity and electronics has opened the door to rewarding careers for multitudes of people. The words electricity and electronics are part of everyone’s vocabulary, yet a surprising number of people fail to make the proper distinction between these two words. As you begin your studies of these subjects, you should know how to differentiate between the two. |
|
To begin, this is a book about electricity, not electronics. The study of electricity precedes the study of electronics. No one can hope to learn the concepts of electronics without having first mastered the principles of electricity. Then how do these two terms differ from each other? |
|
Electricity is best thought of as a form of energy. Natural energy, of course, manifests itself in many different forms of which electricity is but one example. |
|
You may recall one of the cardinal rules of science, which states that energy can neither be created nor destroyed; thus, mankind cannot create electricity. All we can do is produce and utilize electricity by converting various forms of energy. |
|
Let us consider, by contrast, the word electronics. Electronics deals with specific applications of electrical principles that are earmarked by the following characteristics: |
|
1 Electronics refers to the processing of informational signals. In other words, an electronic device is designed to convey, collect, or transmit informational data in the form of small variations in electrical voltages or currents. 2 Electronic equipment utilizes components such as electronic tubes or semiconductor devices. 3 The electronic signal does not necessarily require the use of metal conductors. The electrical energy may be wireless, or transmitted through space. |
|
1–2 WHY THIS BOOK IS CALLED DIRECT CURRENT FUNDAMENTALS |
|
Electricity is available to the consumer in two different forms: direct current (DC) and alternating current (AC). Of the two, alternating current is the more prevalent form. This kind of electricity is commercially generated and distributed by public utilities. |
|
AC is available in two different versions: (1) polyphase AC, which is mainly used for industrial and commercial applications, and (2) single-phase AC, which is used in the home as well as in commerce. |
|
Direct current, by contrast, is not commercially available to the average consumer. It is used in batteries, such as in mobile equipment; in all electronic devices; and for special industrial applications, such as adjustable speed drives and electroplating. |
|
You may be curious about the difference between DC and AC. DC sources are distinguished by a fixed polarity, such as in a car battery, which has two terminals, one marked positive and one marked negative. Current from DC sources flows steadily in one direction only. |
|
AC sources do not have such polarity markings. Just think of an electrical wall outlet in your home. Current from such sources changes direction continually, flowing back and forth in a conductor. |
|
As stated before, direct current generally is not commercially available. If needed, it may be locally provided by use of: |
|
• DC generators • Batteries • Rectifiers (devices for the conversion of AC into DC) |
|
This book, then, is concerned with the study and application of DC principles. One might ask: “Why begin our studies with DC instead of the more common AC?” The reason is that DC fundamentals are easier for the beginning student and, once learned, will afford an easy transition to AC fundamentals. |
|
1–3 EARLY HISTORY OF ELECTRICITY |
|
Our knowledge of electricity has been gathered over the years by experimenters in many areas: magnetism; batteries; current, through gases and through vacuum; and studies of metals, heat, and light. Some of the simplest and most important ideas were discovered fairly recently. These recently discovered facts will be used in this discussion because they will be helpful in gaining an easier understanding of electricity. |
|
The first written records describing electrical behavior were made 2,500 years ago. These records show that the Greeks knew that amber rubbed on cloth attracted feathers, cloth fibers, and other lightweight objects. The Greek name for amber was elektron. From elektron came our word electric, which at first meant “being like amber,” or, in other words, having the property of attraction. |
|
A hard rubber comb and the plastic case of a pen both acquire a strange ability after being rubbed on a coat sleeve—the ability to attract other objects. Long ago, the name charging was given to the rubbing process that gives the plastic or hard rubber its ability to attract. After rubbing, the object was said to be charged. The charge given to the object was thought to be an invisible load of electricity. |
|
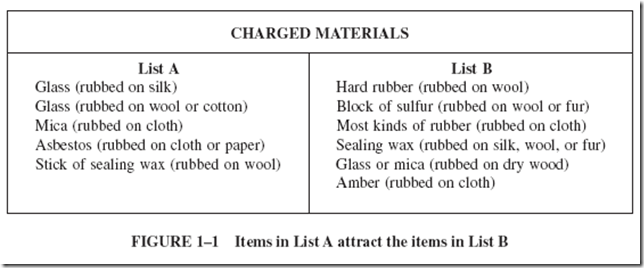
About 300 years ago, a few men began a systematic study of the behavior of various charged objects. They soon found that repulsion was just as important as attraction. Their experiments showed that charged materials could be divided into the two groups shown in Figure 1–1. |
|
Any item from List A attracts any item from List B and vice versa. (Charged glass attracts charged rubber and vice versa.) • Any item in List A repels any other item in List A. (Charged glass repels charged mica.) • Any item in List B repels any other item in List B. (Charged rubber repels charged rubber.) |
|
These results illustrate the law of attraction and repulsion: |
|
Unlike charges attract; like charges repel. |
|
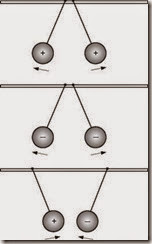
Various names were suggested to describe List A and List B. They could have been called by any pair of opposite-sounding names: Up and Down, or Black and White. The pair of names finally accepted by scientists was suggested by Benjamin Franklin: Positive for List A, Negative for List B. The first item in each list was used as a standard and led to the original definition of the terms positive and negative: Anything that repels charged glass is like charged glass and has a positive charge; anything that repels charged rubber is like charged rubber and has a negative charge, as shown in Figure 1–2. |
|
FIGURE 1–2 Unlike charges attract each other and like charges repel each other |
|
The frictional movement involved in rubbing the objects together is not of vital importance. Hard rubber simply pressed against wool (no rubbing) and then removed will get its negative charge although not as strongly as if it were rubbed. The only value of the rubbing is to bring the rubber into contact with more of the surface area of the wool fibers. |
|
For a further understanding of what is occurring in materials when they are electrically charged, we need to review some facts about the internal structure and composition of all materials. |
|
1–4 ONE HUNDRED ELEMENTS—BUILDING BLOCKS OF NATURE |
|
All of the thousands of kinds of materials on the Earth consist of various combinations of simple materials called elements. Carbon, oxygen, copper, iron, zinc, tin, chlorine, aluminum, gold, uranium, neon, lead, silver, nitrogen, and hydrogen are elements that most of us have heard of or have used. We do not often use the elements silicon, calcium, and sodium in the pure form, so their names may be less familiar. However, these three elements in combination with oxygen and other elements make up the largest part of the soil and rocks of our Earth and help form many manufactured products of everyday use. |
|
There are more than 100 elements. Some of them we never hear of, either because they are very scarce or because people have not yet developed industrial uses for them. Because germanium, beryllium, and titanium are now being used in the electronics and aircraft manufacturing industries, their names are more familiar than they were a few years ago, whereas in 1890 few people had heard of aluminum because it was then a rare and precious metal. |
|
Since there are over 100 different elements, there are over 100 different kinds of atoms. The word atom is the name for the smallest particle of an element. We can talk about atoms of carbon, oxygen, and copper because these materials are elements. Single atoms are so small that there is no use wondering what one atom looks like. For example, it is estimated that there are about 30,000,000,000,000,000,000,000 atoms of copper in a penny and that the penny is about six million atoms thick. If an imaginary slicing machine sliced a penny into six million slices of copper, each slice one atom thick, then each slice would contain five million billion atoms. |
|
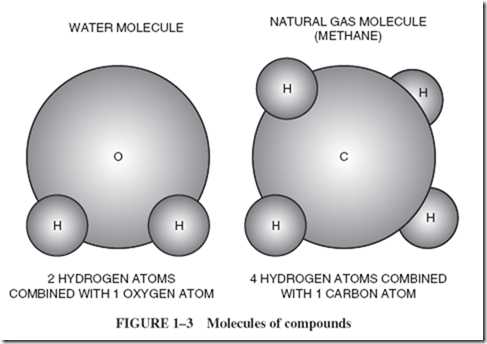
We do not talk about an atom of water, because water is not an element; it is a compound. The smallest possible speck of a compound is properly called a molecule, Figure 1–3. Each molecule of water is made of two atoms of hydrogen and one atom of oxygen. The word compound is the name for a material composed of two or more different elements combined. Water is a compound, and the smallest particle of water is a molecule. |
|
1–5 THE ATOM ANALYZED—ELECTRONS, PROTONS, AND NEUTRONS |
|
All of the more than 100 kinds of atoms are found to consist of still smaller particles. These particles are so completely different from any known material that any imaginative picture of them is sure to be inaccurate. |
|
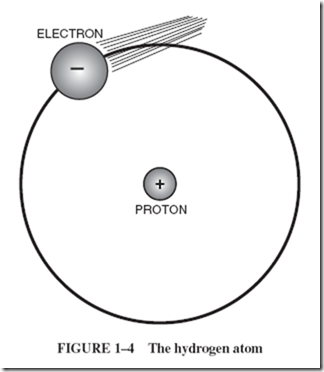
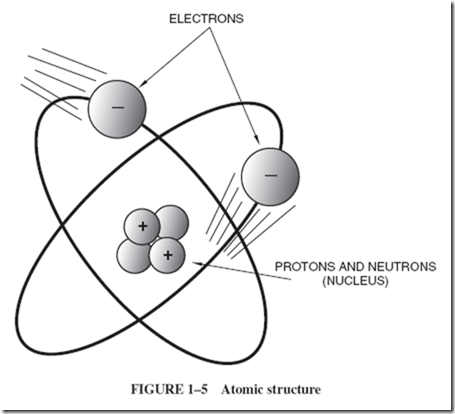
Atoms of hydrogen gas are the simplest in structure of all atoms. Hydrogen atoms consist of a single positively charged particle in the center, with one negatively charged particle whizzing around it at high speed. The positively charged particle has been given the name proton; the negatively charged particle is called an electron. |
|
Figure 1–4 is not drawn to scale because the diameter of the atom is several thousand times greater than the diameters of the particles in it. To show relative dimensions, a more exact representation would have a pinhead-sized electron revolving in an orbit |
|
150 feet across. However, the pinhead is not an exact representation either, for the electron is highly indefinite in shape. It is more like a fuzzy wisp that ripples, spins, and pulses as it rotates around the proton in the center. The mathematical equation that describes it best is the equation that describes a wave. An atom has no outer skin other than the surface formed by its whirling electrons. This is a repelling surface, comparable to the whirling “surface” that surrounds a child skipping a rope. There is as much relative open space within the atom as there is in our solar system. |
|
The proton that forms the center of the hydrogen atoms is smaller than the electron but 1,840 times as heavy. The most important properties of the proton are its positive charge and its weight. The number of protons determines the identity of the element. For example, an atom containing 29 protons must be an atom of copper. |
|
As we look at diagrams of other atoms, we need two new words to describe them. The nucleus of the atom is the name given to the tightly packed, heavy central core where the protons of the atom are assembled. Along with the protons are other particles called neutrons, as shown in Figure 1–5. |
|
The name neutron indicates that this heavy particle is electrically neutral; neutrality and weight are its most important properties. A neutron is probably a tightly collapsed combination of an electron and a proton. |
|
At first, it may be hard to realize that these three particles—electrons, protons, and neutrons—make up all materials. All electrons are alike, regardless of the material from which they come or in which they exist; see Figure 1–6. All protons are alike, regardless of the material in which they exist. Neutrons, too, are all alike. |
|
1–6 THE ATOMIC THEORY—CORNERSTONE OF ELECTRICAL THEORY |
|
In 1808, a scientist named John Dalton proposed that all matter was composed of atoms. Although the assumptions that Dalton used to prove his theory were later found to be factually incorrect, the idea that all matter is composed of atoms was adopted by most of the scientific world. Then, in 1897, J.J. Thompson discovered the electron. Thompson determined that electrons have a negative charge and that they have very little mass compared to the atom. He proposed that atoms have a large, positively charged massive body with negatively charged electrons scattered throughout it. Thompson also proposed that the negative charge of the electrons exactly balanced the positive charge of the large mass, causing the atom to have a net charge of zero. Thompson’s model of the atom proposed that electrons existed in a random manner within the atom, much like firing BB’s from a BB gun into a slab of cheese. This was referred to as the “plum pudding model” of the atom. |
|
In 1913, Neils Bohr, a Danish scientist, presented the most accepted theory concerning the structure of an atom. In the Bohr model, electrons exist in specific or “allowed” orbits around the nucleus in much the same way that planets orbit the sun. The orbit in which the electron exists is determined by the electron’s mass times its speed times the radius of the orbit. These factors must equal the positive force of the nucleus. In theory there can be an infinite number of allowed orbits. |
|
When an electron receives enough energy from some other source it “quantum jumps” into a higher allowed orbit. Electrons, however, tend to return to a lower allowed orbit. When this occurs, the electron emits the excess energy as a single photon of electromagnetic energy. |
|
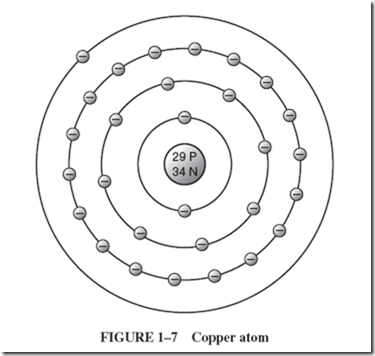
The arrangement of electrons around the nucleus determines most of the physical and chemical properties and the behavior of the element. The electrons of the atom are often pictured in distinct layers, or shells, around the nucleus. The innermost shell of electrons contains no more than 2 electrons. The next shell contains no more than 8 electrons; the third, no more than 18; and the fourth, 32. Let us consider the model of a copper atom shown in Figure 1–7. |
|
The 29 electrons of the copper atom are arranged in four layers, or shells: 2 in the shell nearest the nucleus, 8 in the next, and 18 in the third, for a total of 28 electrons. The single 29th electron circulates all alone in the fourth shell. |
|
This outermost shell is known as the valence shell, and electrons occupying this orbit are known as valence electrons. When energy is applied to a valence electron, it may dislodge itself from its parent atom and is then known as a free electron. In this position (where it is relatively far from the positive nucleus and is screened from its attracting positive charge by the other electrons), this single electron is not tightly held to the atom and is fairly free to travel. |
|
If we examine the electron arrangement in all kinds of atoms, we find that most of them have one, two, or three electrons in an outer shell, shielded from the positive nucleus by one or more inner shells of electrons. These elements are all called metals. Metals are fairly good conductors of electricity because they have many free electrons that can move from atom to atom. |
|
Elements with five, six, or seven electrons in their outermost ring are classified as nonmetals. Diagrams of two such nonmetallic elements, sulfur and iodine, are shown in Figure 1–8. They are not good conductors for the following reasons: |
|
1 Their outside electrons are not as well shielded from the attracting force of the nucleus because the atom has relatively fewer electrons in the inside shells helping to screen any individual outer electron from the attracting force of the nucleus. 2 A shell of eight electrons has a degree of energy stability. Atoms with seven, six, or five electrons in the outer shell will readily pick up and hold the one, two, or three electrons that will build the shell up to eight. |
|
For example, if we try to push some electrons through a block of sulfur, we find that our electrons drop into the empty spaces in the outer shells of the sulfur atoms and are stuck there. This stable shell of eight electrons leaves sulfur with no free electrons ready to slide over to the next atom and with no room for a newcomer. |
|
The word ion refers to an electrically unbalanced atom. Considering this statement, it may be concluded that a positive ion is an unbalanced atom that has lost some of its electrons, and conversely, a negative ion is an unbalanced atom that has gained some electrons. |
|
SUMMARY |
|
• Electricity is a form of energy. |
|
• Electronics deals with specific applications of electrical principles. |
|
• Electrical systems may be classified as being either direct current (DC) or alternating current (AC). |
|
• AC can be converted into DC by the use of rectifiers. |
|
• Unlike charges attract; like charges repel. |
|
• An element is a single uncombined substance consisting of only one kind of atom. An atom is the smallest portion of an element. |
|
• A compound is a substance that can be chemically separated into two or more elements. A molecule is the smallest portion of a compound. |
|
• Atoms consist of various numbers of electrons, protons, and neutrons. |
|
• Electrons are negatively charged and lightweight and move outside the nucleus. |
|
• Electrons are arranged in layers, or shells, around the nucleus of the atom. |
|
• The number of electrons in the outer shell of the atom determines most of the electrical properties of the element. |
|
• Protons are positively charged, are heavy, and are contained within the nucleus. |
|
• Neutrons are not charged, are heavy, and are contained within the nucleus. |
|
• The number of protons determines the kind of element. |
|
• A negatively charged object is one that has gained extra electrons. |
|
• A positively charged object is one that has lost some of its electrons. |
|
• Electricity is explained by the behavior of electrons. |
|
• Substances with many free electrons are classified as conductors. |
|
• Substances with very few free electrons are classified as insulators. |
|
• All materials can become electrically charged. |
|
• The motion of electrons through a material is called the electric current. |
|
• Electrically balanced atoms are called ions. |
|
• A positive ion is an atom that has lost one or more of its valence electrons. |
|
• A negative ion is an atom that has gained one or more of its valence electrons. |
|
Achievement Review |
|
1. Using our knowledge of electrons, how do we now define the terms positive charge and negative charge? |
|
2. Using what you know of electron theory, explain what must happen to give an object a positive charge. What happens to give an object a negative charge? |
|
3. State the law of attraction and repulsion. |
|
4. What kind of charge does an electron have? |
|
5. Would two electrons repel or attract each other? Explain. |
|
6. What do each of these words mean: atom, element, molecule, compound, proton, electron, neutron? (There is no point in memorizing definitions of such terms; you should try to understand their meaning so that you can use them correctly.) |
|
7. Tell how atoms of metals differ from atoms of nonmetals in their electron arrangement. Why are metals good conductors? |
|
8. There is an element called gallium. Its atoms have 31 electrons. Referring to the picture of a copper atom, Figure 1–7, how would you expect the electrons of an atom of gallium to be arranged? Is gallium a metal? |
|
9. Explain the terms AC and DC. Tell how they differ from each other. |
|
10. Complete the following sentences. • All materials consist of over 100 simple substances called __________. • The smallest particles of these simple substances are called __________ . • Atoms consist of three still smaller particles called __________ , __________ , and __________ . Of these three, the one with least weight is the __________ ; the one most readily movable is the __________ ; the positively charged particle is the __________ ; the negatively charged particle is the __________ ; the particle most responsible for the electrical behavior of materials is the __________ . An atom with unbalanced electrical charges is known as a(n) __________ . An atom with a surplus of electrons is said to be a __________ ion, and if it has a deficiency of electrons, it is called a __________ ion. |