1.6 Density, Specific Volume, and Specific Weight
By Eq. (1.1), density is mass per unit volume; by Eq. (1.3), specific volume is volume per unit mass. By comparing their definitions, we see that the two properties are related by
v = 1/ρ (1.6)
Associated with (mass) density is weight density, or specific weight g :
γ=W/ V (1.7)
with units N/m3 (lbf/ft3). (Note that g is volume-specific, not mass-specific.) Specific weight is related to density through W = mg:
y=mg/mv=pg (1.8)
For water, nominal values of r and g are, respectively, 1000 kg/m3 and 9810 N/m3. For air at standard conditions, the nominal values are 1.21 kg/m3 and 11.86 N/m3.
EXAMPLE 1.3
The mass of air in a room 3 m × 5 m × 20 m is known to be 350 kg. Determine
the density, specific volume, and specific weight of the air.
Solution
Equations (1.1), (1.6), and (1.8) are used: 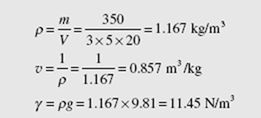
In gases and liquids, the effect of a normal force acting on an area is the pressure. If a force ΔF acts at an angle to an area Δ A (Fig. 1.7), only the normal component Δ Fn enters into the definition of pressure:
The SI unit of pressure is the pascal (Pa), where 1 Pa = 1 N/m2. The pascal is a relatively small unit so pressure is usually measured in kPa. By considering the pressure
forces acting on a triangular fluid element of constant depth we can show that the pressure at a point in a fluid in equilibrium is the same in all directions; it is a scalar quantity. For gases and liquids in relative motion, the pressure may vary from point to point, even at the same elevation; but it does not vary with direction at a given point.
PRESSURE VARIATION WITH ELEVATION
In the atmosphere, pressure varies with elevation. This variation can be expressed mathematically by summing vertical forces acting on an infinitesimal element of
air. The force PA on the bottom of the element and (P + dP)A on the top balance the
weight pgAdz to provide
dP = −rgdz (1.10)
If r is a known function of z, the above equation can be integrated to give P(z) 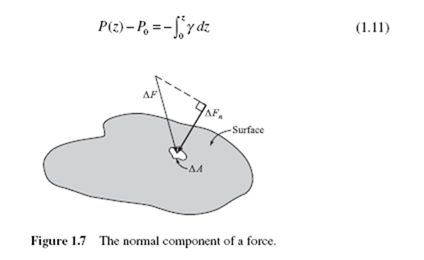
where we used rg = g. For a liquid, g is constant. If we write Eq. (1.10) using dh = −dz, we have
dP = g dh (1.12)
where h is measured positive downward. Integrating this equation, starting at a liquid surface where usually P = 0, results in
P = g h (1.13)
This equation can be used to convert a pressure to pascals when that pressure is measured in meters of water or millimeters of mercury.
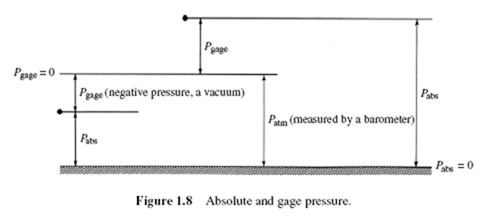
In many relations, absolute pressure must be used. Absolute pressure is gage pressure plus the local atmospheric pressure:
Pabs = Pgage + Patm (1.14)
A negative gage pressure is often called a vacuum, and gages capable of reading negative pressures are vacuum gages. A gage pressure of −50 kPa would be referred
to as a vacuum of 50 kPa (the sign is omitted).
Figure 1.8 shows the relationships between absolute and gage pressure at two different points.
The word “gage” is generally specified in statements of gage pressure, e.g., p = 200 kPa gage. If “gage” is not mentioned, the pressure will, in general, be an absolute pressure. Atmospheric pressure is an absolute pressure, and will be taken as 100 kPa (at sea level), unless otherwise stated. It is more accurately 101.3 kPa at standard conditions. It should be noted that atmospheric pressure is highly dependent
on elevation; in Denver, Colorado, it is about 84 kPa; in a mountain city with elevation 3000 m, it is only 70 kPa. In Table B.1, the variation of atmospheric pressure with elevation is listed.
EXAMPLE 1.4
Express a pressure gage reading of 20 mm Hg in absolute pascals at an elevation of 2000 m. Use gHg = 13.6ywater.
Solution
First we convert the pressure reading into pascals. We have ![]()
To find the absolute pressure we simply add the atmospheric pressure to the above value. Referring to Table B.1, Patm = 0.7846 × 101.3 = 79.48 kPa. The absolute pressure is then
P = Pgage + Patm = 2.668 + 79.48 = 82.15 kPa
Note: We express an answer to either 3 or 4 significant digits, seldom, if ever, more than 4. Information in a problem is assumed known to at most 4 significant digits. For example, if the diameter of a pipe is stated as 2 cm, it is assumed that it is 2.000 cm. Material properties, such as density or a gas constant, are seldom known to even 4 significant digits. So, it is not appropriate to state an answer to more than 4 significant digits.
EXAMPLE 1.5
A 10-cm-diameter cylinder contains a gas pressurized to 600 kPa. A frictionless piston is held in position by a stationary spring with a spring constant of 4.8 kN/m. How much is the spring compressed?
Solution
The pressure given is assumed to be absolute (this is the case unless otherwise stated). A force balance on the piston provides
PA − Patm A = Kspring Δ x
(600 000 − 100 000)π × 0.052 = 4800Δ x ∴ Δ x = 0.818 m
Make sure you check the units. Pressure typically has units of pascals when input into equations and area has units of square meters.
1.8 Temperature
Temperature is actually a measure of molecular activity. However, in classical thermodynamics the quantities of interest are defined in terms of macroscopic observations only, and a definition of temperature using molecular measurements is not useful. Thus we must proceed without actually defining temperature. What we shall do instead is discuss equality of temperatures.
EQUALITY OF TEMPERATURES
Let two bodies be isolated from the surroundings but placed in contact with each other. If one is hotter than the other, the hotter body will become cooler and the cooler body will become hotter; both bodies will undergo change until all properties (e.g., pressure) of the bodies cease to change. When this occurs, thermal equilibrium is said to have been established between the two bodies. Hence, we state that two systems have equal temperatures if no change occurs in any of their properties when the systems are brought into contact with each other. In other words, if two systems are in thermal equilibrium, their temperatures are postulated to be equal.
A rather obvious observation is referred to as the zeroth law of thermodynamics: if two systems are equal in temperature to a third, they are equal in temperature to each other.
RELATIVE TEMPERATURE SCALE
To establish a temperature scale, we choose the number of subdivisions, called degrees, between the ice point and the steam point. The ice point exists when ice and water are in equilibrium at a pressure of 101 kPa; the steam point exists when liquid water and its vapor are in a state of equilibrium at a pressure of 101 kPa. On the Fahrenheit scale there are 180o between these two points; on the Celsius scale, 100o. On the Fahrenheit scale the ice point is assigned the value of 32 and on the Celsius scale it is assigned the value 0. These selections allow us to write
Tc = (5/9) (TF − 32) (1.15)
ABSOLUTE TEMPERATURE SCALE
The second law of thermodynamics will allow us to define an absolute temperature scale; however, since we have not introduced the second law at this point and we have
immediate use for absolute temperature, an empirical absolute temperature scale will be presented. The relations between absolute and relative temperatures are
TK = TC + 273.15 (1.16)
The value 273 is used where precise accuracy is not required, which is the case for most engineering situations. The absolute temperature on the Celsius scale is given in kelvins (K). Note: We do not use the degree symbol when writing kelvins,e.g., T1= 400 K.
1.9 Energy
A system may possess several different forms of energy. Assuming uniform proper- ties throughout the system, its kinetic energy is given by
KE = 1/2 mV 2 (1.17)
where1 V is the velocity of each particle of substance, assumed constant over the entire system. If the velocity is not constant for each particle, then the kinetic energy is found by integrating over the system.
The energy that a system possesses due to its elevation h above some arbitrarily selected datum is its potential energy; it is determined from the equation
PE = mgh (1.18)
Other forms of energy include the energy stored in a battery, energy stored in an electrical condenser, electrostatic potential energy, and surface energy. In addition, there is the energy associated with the translation, rotation, and vibration of the molecules, electrons, protons, and neutrons, and the chemical energy due to bonding between atoms and between subatomic particles. All of these forms of energy will be referred to as internal energy and designated by the letter U. In combustion, energy is released when the chemical bonds between atoms are rearranged. In this book, our attention will be primarily focused on the internal energy associated with the motion of molecules, i.e., temperature. In Chap. 9, the combustion process is presented.
Internal energy, like pressure and temperature, is a property of fundamental importance. A substance always has internal energy; if there is molecular activity,
there is internal energy. We need not know, however, the absolute value of internal energy, since we will be interested only in its increase or decrease.
We now come to an important law, which is often of use when considering isolated systems. The law of conservation of energy states that the energy of an isolated system remains constant. Energy cannot be created or destroyed in an isolated system; it can only be transformed from one form to another. This is expressed as
KE + PE + U = const or 1/2 mV 2 + mgh + U = const (1.19)
Consider a system composed of two automobiles that hit head on and are at rest after the collision. Because the energy of the system is the same before and after the collision, the initial total kinetic energy KE must simply have been transformed into another kind of energy, in this case, internal energy U, stored primarily in the deformed metal.
EXAMPLE 1.6
A 2200-kg automobile traveling at 90 km/h (25 m/s) hits the rear of a stationary, 1000-kg automobile. After the collision the large automobile slows to 50 km/h
(13.89 m/s), and the smaller vehicle has a speed of 88 km/h (24.44 m/s). What has been the increase in internal energy, taking both vehicles as the system?
Solution
The kinetic energy before the collision is (V = 25 m/s)
KE1 = 1/2 =maVa 2= (1/2) × 2200 × 252 = 687 500 J
where the subscript a refers to the first automobile; the subscript b refers to the second one. After the collision the kinetic energy is 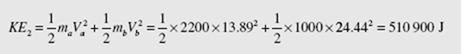
The conservation of energy requires that
E1 = E2 or KE1 + U1 = KE2 + U2
Thus,
U2 − U1 = KE1 − KE2 = 687 500 − 510 900 = 176 600 J or 176.6 kJ
Quiz No. 1
1. Engineering thermodynamics does not include energy
(A) transfer
(B) utilization k
(C) storage
(D) transformation
2. Which of the following would be identified as a control volume?
(A) Compression of the air-fuel mixture in a cylinder
(B) Filling a tire with air at a service station
(C) Compression of the gases in a cylinder
(D) The flight of a dirigible
3. Which of the following is a quasiequilibrium process?
(A) Mixing a fluid
(B) Combustion
(C) Compression of the air-fuel mixture in a cylinder
(D) A balloon bursting
4. The standard atmosphere in meters of gasoline (g = 6660 N/m3) is nearest
(A) 24.9 m
(B) 21.2 m
(C) 18.3 m
(D) 15.2 m
5. A gage pressure of 400 kPa acting on a 4-cm-diameter piston is resisted by a spring with a spring constant of 800 N/m. How much is the spring compressed? Neglect the piston weight and friction.
(A) 63 cm
(B) 95 cm
(C) 1.32 m
(D) 1.98 m
6. Which of the following processes can be approximated by a quasiequilibrium process?
(A) The expansion of combustion gases in the cylinder of an automobile engine
(B) The rupturing of a balloon
(C) The heating of the air in a room with a radiant heater
(D) The cooling of a hot copper block brought into contact with ice cubes
7. Determine the weight of a mass at a location where g = 9.77 m/s2 (on the
top of Mt. Everest) if it weighed 40 N at sea level.
(A) 39.62 N
(B) 39.64 N
(C) 39.78 N
(D) 39.84 N
8. Determine g if g = 9.81 m/s2, V = 10 m3, and v = 20 m3/kg.
(A) 2.04 N/m3
(B) 1.02 N/m3
(C) 0.49 N/m3
(D) 0.05 N/m3
9.If Patm= 100 kPa, the pressure at a point where the gage pressure is 300 mmHg is nearest (g Hg = 13.6 gwater)
(A) 40 kPa
(B) 140 kPa
(C) 160 kPa
(D) 190 kPa
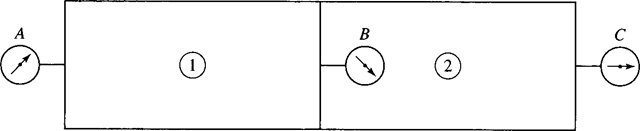
10. A large chamber is separated into compartments 1 and 2, as shown, that are kept at different pressures. Pressure gage A reads 400 kPa and pressure gage B reads 180 kPa. If the barometer reads 720 mmHg, determine the absolute pressure of C.
(A) 320 kPa
(B) 300 kPa
(C) 280 kPa
(D) 260 kPa
11. A 10-kg body falls from rest, with negligible interaction with its surroundings (no friction). Determine its velocity after it falls 5 m.
(A) 19.8 m/s
(B) 15.2 m/s
(C) 12.8 m/s
(D) 9.9 m/s
12. The potential energy stored in a spring is given by Kx2/2, where K is the spring constant and x is the distance the spring is compressed. Two springs are designed to absorb the kinetic energy of an 1800-kg vehicle. Determine the spring constant necessary if the maximum compression is to be 100 mm for a vehicle speed of 16 m/s.
(A) 23 MN/m
(B) 25 MN/m
(C) 27 MN/m
(D) 29 MN/m
Quiz No. 2
1. In a quasiequilibrium process, the pressure
(A) remains constant
(B) varies with location
(C) is everywhere constant at an instant
(D) depends only on temperature
2. Which of the following is not an extensive property?
(A) Momentum
(B) Internal energy
(C) Temperature
(D) Volume
3. The joule unit can be converted to which of the following?
(A) kg · m2/s
(B) kg · m/s2
(C) Pa · m3
(D) Pa/m2
4. Convert 178 kPa gage of pressure to absolute millimeters of mercury (phg = 13.6prwater).
(A) 2080 mm
(B) 1820 mm
(C) 1640 mm
(D) 1490 mm
5. Calculate the pressure in the 240-mm-diameter cylinder shown. The spring is compressed 60 cm. Neglect friction.
(A) 198 kPa
(B) 135 kPa
(C) 110 kPa
(D) 35 kPa
6. A cubic meter of a liquid has a weight of 9800 N at a location where g = 9.79 m/s2. What is its weight at a location where g = 9.83 m/s2?
(A) 9780 N
(B) 9800 N
(C) 9820 N
(D) 9840 N
7. Calculate the force necessary to accelerate a 900-kg rocket vertically upward at the rate of 30 m/s2.
(A) 18.2 kN
(B) 22.6 kN
(C) 27.6 kN
(D) 35.8 kN
8. Calculate the weight of a body that occupies 200 m3 if its specific volume is 10 m3/kg.
(A) 20 N
(B) 92.1 N
(C) 132 N
(D) 196 N
9. The pressure at a point where the gage pressure is 70 cm of water is nearest
(A) 169 kPa
(B) 107 kPa
(C) 69 kPa
(D) 6.9 kPa
10. A bell jar 200 mm in diameter sits on a flat plate and is evacuated until a vacuum of 720 mmHg exists. The local barometer reads 760 mmHg.
Estimate the force required to lift the jar off the plate. Neglect the weight of the jar.
(A) 3500 N
(B) 3000 N
(C) 2500 N
(D) 2000 N
11. An object that weighs 4 N traveling at 60 m/s enters a viscous liquid and is essentially brought to rest before it strikes the bottom. What is the increase in internal energy, taking the object and the liquid as the system? Neglect the potential energy change.
(A) 734 J
(B) 782 J
(C) 823 J
(D) 876 J
12. A 1700-kg vehicle traveling at 82 km/h collides head-on with a 1400-kg vehicle traveling at 90 km/h. If they come to rest immediately after impact, determine the increase in internal energy, taking both vehicles as the system.
(A) 655 kJ
(B) 753 kJ
(C) 879 kJ
(D) 932 kJ