Composition of radiant heat
Both conduction and convection are ways of carrying heat from one place to another which require the presence of a material substance, either solid, liquid or gas.
 |
| Greenhouse |
There is a third process of heat transmission which does not require a material medium. This is called radiation, and is the same means by which energy travels from the sun across the empty space beyond earth’s atmosphere.
Radiant heat consists of invisible electromagnetic waves which are able to pass through a vacuum. These waves are partly reflected and partly absorbed by objects on which they fall. The part which is absorbed becomes converted into heat.
Radiant heat which has passed through a vacuum can easily felt by holding the hand near to a vacuum-filled electric lamp when the current is switched on.
The detection of radiant heat. The thermopile
Radiant heat may be detected by converting the heat energy into electric energy. A simple experiment serves to show how this is done.
 |
| Thermoelectric effect |
A copper and an iron wire are twisted together to form a junction, while the free ends of the wires are connected to the terminals of a sensitive reflecting galvanometer. On warming the junction an electric current is produced in the circuit and the galvanometer gives a deflection. This is called the thermoelectric effect.
Thermopile construction
Bismuth and antimony are two metals which show the thermoelectric effect in a marked degree, and they are used for the detection of radiant heat in an instrument called a thermopile. In order to magnify the effect, as many as 64 pairs of antimony and bismuth bars are joined in series, to give 64 junctions on which the radiant heat is allowed to fall. The bars are placed side by side, insulated from one another by paper, and their ends are soldered together.
The whole is mounted in plaster of pairs set in a short brass cylinder, and provided with two terminals connected to the free ends of the two ends bars.
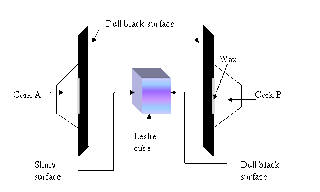 |
| Leslie’s cube experiment |
John Leslie’s experiment: To compare the radiation from different surfaces
The rate at which a body radiates heat depends on its temperature and the nature and area of its surface.
It is found that, for a given temperature, a body radiates most heat when its surface is dull black and least when its surface is highly polished.
Leslie used a hollow copper cube, each side of which had a different surface. One may be highly polished metal, another coated with lamp black by holding it in the flame of a candle, while the remaining two surfaces may be painted in a light and dark color respectively.
In each case the steady deflection obtained on the galvanometer is recorded. The results show that the dull black surface produces the largest, and the polished metal the smallest deflection. Of the painted surfaces, the darker one is usually better, but this is not always the case. The texture of the surface appears to be a more important factor than its color.
Absorption of radiant heat by a surface
As we stated earlier, radiant heat falling on a surface is partly absorbed and partly reflected.
The absorbing powers of a dull black and a polished surface may be compared by two sheets of tinplate, one polished and the other painted dull black. On the reverse side of each plate, a cork is fixed by means of a little melted paraffin wax. The plates are then set up vertically, a short distance apart, with a Bunsen burner midway between.
The better radiator is also the better absorber of heat.
Practical applications of radiation
The investigations on radiation and absorption described above have a number of useful applications.
Buildings which are white-washed or painted in light colors keep cooler in summer, since the light surfaces reflect radiant heat from the sun.
Many factory roofs are aluminium-painted. The bright surface reduces the heat lost in winter, and keeps the interior cool in summer.
We ourselves choose light-colored clothing in summer for the same reason, and in very hot countries white clothing is generally the rule.
The so-called radiators of a hot water central heating system do, in fact, emit most of their heat by convection. Nevertheless, in order to increase the proportion of heat radiated they are sometimes painted in a dark color.
The vacuum flask to store liquefied gases – Thermos
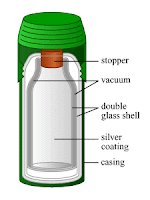 |
| Vacuum flask |
A heat trap
Anyone who walks into a greenhouse, even on a day when the sunlight is rather dull, realizes how efficiently it acts as a heat trap.
