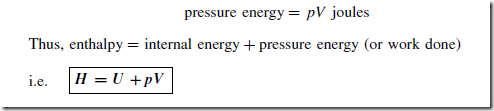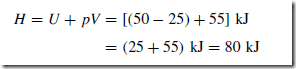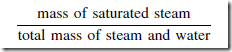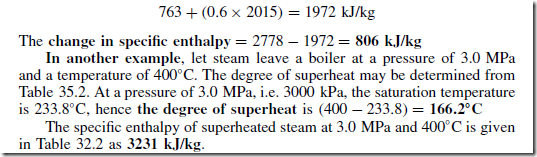Properties of Water and Steam
Principle of Conservation of Energy
When two systems are at different temperatures, the transfer of energy from one system to the other is called heat transfer. For a block of hot metal cooling in air, heat is transferred from the hot metal to the cool air.
The principle of conservation of energy may be stated as
energy cannot be created nor can it be destroyed
and since heat is a form of energy, this law applies to heat transfer problems.
A more convenient way of expressing this law when referring to heat transfer problems is:
Internal Energy
Fluids consist of a very large number of molecules moving in random directions within the fluid. When the fluid is heated, the speeds of the molecules are increased, increasing the kinetic energy of the molecules. There is also an increase in volume due to an increase in the average distance between molecules, causing the potential energy of the fluid to increase. The internal energy, U, of a fluid is the sum of the internal kinetic and potential energies of the molecules of a fluid, measured in joules. It is not usual to state the internal energy of a fluid as a particular value in heat transfer problems, since it is normally only the change in internal energy that is required.
The amount of internal energy of a fluid depends on:
(a) the type of fluid; in gases the molecules are well separated and move with high velocities, thus a gaseous fluid has higher internal energy than the same mass of a liquid
(b) the mass of a fluid; the greater the mass, the greater the number of molecules and hence the greater the internal energy
(c) the temperature; the higher the temperature the greater the velocity of the molecules
Enthalpy
The sum of the internal energy and the pressure energy of a fluid is called the enthalpy of the fluid, denoted by the symbol H and measured in joules. The product of pressure p and volume V gives the pressure energy, or work done, i.e.
As for internal energy, the actual value of enthalpy is usually unimportant and it is the change in enthalpy that is usually required. In heat transfer problems involving steam and water, water is considered to have zero enthalpy at a standard pressure of 101 kPa and a temperature of 0°C. The word ‘specific’ associated with quantities indicates ‘per unit mass’. Thus the specific enthalpy is obtained by dividing the enthalpy by the mass and is denoted by the symbol h. Thus:
The units of specific enthalpy are joules per kilogram (J/kg)
For example, in a closed system, that is, a system in which the mass of fluid remains a constant, the internal energy changes from 25 kJ to 50 kJ and the work done by the system is 55 kJ. The heat transferred to the system to effect this change is given by:
That is, the heat transferred to the system is 80 kJ
Sensible Heat
The specific enthalpy of water, hf , at temperature 8°C is the quantity of heat needed to raise 1 kg of water from 0°C to 8°C, and is called the sensible heat of the water. Its value is given by:
specific heat capacity of water (c) x temperature change
The specific heat capacity of water varies with temperature and pressure but is normally taken as 4.2 kJ/kg, thus
Saturated Steam
When water is heated at a uniform rate, a stage is reached (at 100° C at standard atmospheric pressure) where the addition of more heat does not result in a corresponding increase in temperature. The temperature at which this occurs is called the saturation temperature, tSAT , and the water is called saturated water. As heat is added to saturated water, it is turned into saturated steam. The amount of heat required to turn 1 kg of saturated water into saturated steam is called the specific latent heat of vaporisation, and is given the symbol, hfg . The total specific enthalpy of steam at saturation temperature, hg , is given by:
the specific sensible heat + the specific latent heat of vaporization
Dryness Factor
If the amount of heat added to saturated water is insufficient to turn all the water into steam, then the ratio:
is called the dryness fraction of the steam, denoted by the symbol q. The steam is called wet steam and its total enthalpy h is given by:
Superheated Steam
When the amount of heat added to water at saturation temperature is sufficient to turn all the water into steam, it is called either saturated vapour or dry saturated steam. The addition of further heat results in the temperature of the steam rising and it is then called superheated steam. The specific enthalpy of superheated steam above that of dry saturated steam is given by:
c(tSUP Ł tSAT), where c is the specific heat capacity of the steam and tSUP is the temperature of the superheated steam. The total specific enthalpy of the superheated steam is given by:
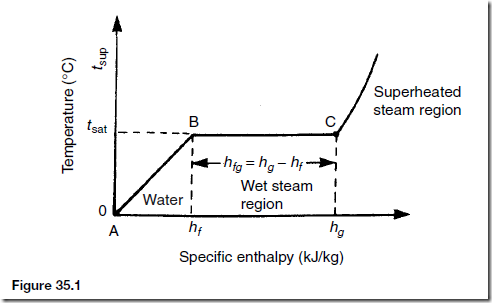
Temperature/Specific Enthalpy Graph
The relationship between temperature and specific enthalpy can be shown graphically and a typical temperature/specific enthalpy diagram is shown in Figure 35.1. In this figure, AB represents the sensible heat region where any increase in enthalpy results in a corresponding increase in temperature. BC is called the evaporation line and points between B and C represent the wet steam region (or latent region), point C representing dry saturated steam. Points to the right of C represent the superheated steam region.
Steam Tables
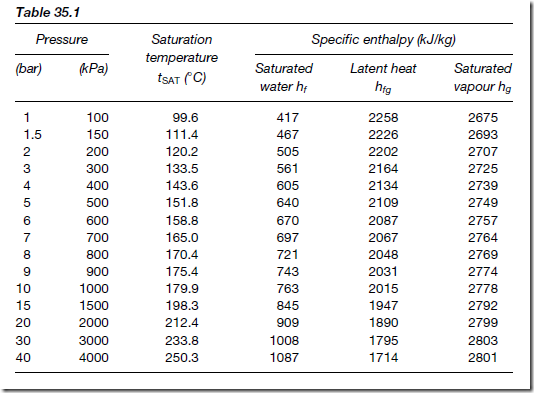
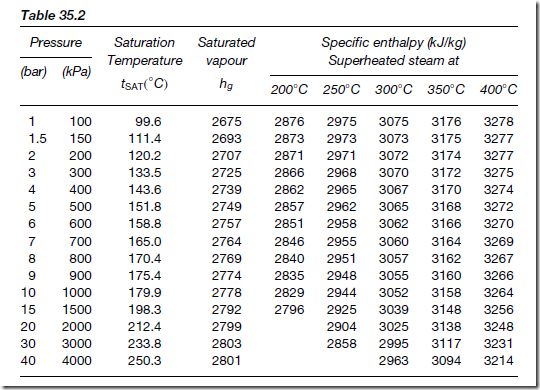
The boiling point of water, tSAT and the various specific enthalpies associated with water and steam [hf , hfg and c(tSUP Ł tSAT)] all vary with pressure. These values at various pressures have been tabulated in steam tables, extracts from these being shown in Tables 35.1 and 35.2
In Table 35.1, the pressure in both bar and kilopascals, and saturated water temperature, are shown in columns on the left. The columns on the right give the corresponding specific enthalpies of water (hf) and dry sat- urated steam (hg ), together with the specific enthalpy of the latent heat of vaporization (hfg ). The columns on the right of Table 35.2 give the specific
enthalpies of dry saturated steam (hg) and superheated steam at various temperatures. The values stated refer to zero enthalpy. However, if the degree of superheat is given, this refers to the saturation temperature. Thus at a pressure of 100 kPa, the column headed, say, 250° C has a degree of superheat of (250 Ł 99.6)°C, that is 150.4° C.
For example, let some dry saturated steam at a pressure of 1.0 MPa be cooled at constant pressure until it has a dryness fraction of 0.6. The change in the specific enthalpy of the steam is determined as follows:
From Table 35.1, the specific enthalpy of dry saturated steam hg , at a pressure of 1.0 MPa (i.e. 1000 kPa) is 2778 kJ/kg. From earlier, the specific enthalpy of wet steam is hf C qhfg . At a pressure of 1.0 MPa, hf is 763 kJ/kg and hfg is 2015 kJ/kg. Thus, the specific enthalpy of the wet steam is given by:
Superheated steam behaves very nearly as if it is an ideal gas and the gas laws introduced in Chapter 34 may be used to determine the relationship between pressure, volume and temperature.