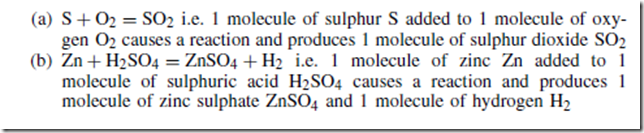Chemical Reactions
Introduction
A chemical reaction is an interaction between substances in which atoms are rearranged. A new substance is always produced in a chemical reaction.
Air is a mixture, and its composition by volume is approximately: nitrogen 78%, oxygen 21%, other gases (including carbon dioxide) 1%.
Oxygen
Oxygen is an odourless, colourless and tasteless element. It is slightly soluble in water (which is essential for fish), has a boiling point of Ð183° C (i.e. 90 K), a freezing point of Ð219° C (i.e. 54 K) and has approximately the same density as air. Oxygen is a strongly active chemical element and combines with many substances when they are heated.
Uses of oxygen include: chemical processing, metal cutting and welding processes to give a very hot flame when burnt with other gases, and for divers, mountaineers, fire-fighters using breathing apparatus and for medical use in hospitals.
If a substance, such as powdered copper, of known mass, is heated in air, allowed to cool, and its mass remeasured, it is found that the substance has gained in mass. This is because the copper has absorbed oxygen from the air and changed into copper oxide. In addition, the proportion of oxygen in the air passed over the copper will decrease by the same amount as the gain in mass by the copper.
All substances require the presence of oxygen for burning to take place. Any substance burning in air will combine with the oxygen. This process is
called combustion, and is an example of a chemical reaction between the burning substance and the oxygen in the air, the reaction producing heat. The chemical reaction is called oxidation.
An element reacting with oxygen produces a compound that contains only atoms of the original element and atoms of oxygen. Such compounds are called oxides. Examples of oxides include: copper oxide CuO, hydrogen oxide H2O (i.e. water) and carbon dioxide CO2
Rusting
Rusting of iron (and iron-based materials) is due to the formation on its surface of hydrated oxide of iron produced by a chemical reaction. Rusting of iron always requires the presence of oxygen and water.
Any iron or steel structure exposed to moisture is susceptible to rusting. This process, which cannot be reversed, can be dangerous since structures may be weakened by it. Examples of damage caused by rusting may be found in steel parts of a motor vehicle, the hull of ships, iron guttering, bridges and similar structures. Rusting may be prevented by:
(i) painting with water-resistant paint
(ii) galvanising the iron
(iii) plating the iron (see chapter 42, page 218)
(iv) an oil or grease film on the surface
Chemical Equations
To represent a reaction a chemical shorthand is used. A symbol represents an element (such as H for hydrogen, O for oxygen, Cu for copper, Zn for zinc, and so on) and a formula represents a compound and gives the type and number of elements in the compound. For example, one molecule of sulphuric acid, H2SO4, contains 2 atoms of hydrogen, 1 atom of sulphur and 4 atoms of oxygen. Similarly, a molecule of methane gas, CH4 , contains 1 atom of carbon and 4 atoms of hydrogen.
The rearrangement of atoms in a chemical reaction is shown by chemical equations using formulae and symbols.
For example:
In a chemical equation:
(i) each element must have the same total number of atoms on each side of the equation; for example, in chemical equation (b) above each side of the equation has 1 zinc atom, 2 hydrogen atoms, 1 sulphur atom and 4 oxygen atoms
(ii) a number written in front of a molecule multiplies all the atoms in that molecule
Acids and Alkalis
An acid is a compound containing hydrogen in which the hydrogen can be easily replaced by a metal. For example, in equation (b) above, it is shown that zinc reacts with sulphuric acid to give zinc sulphate and hydrogen.
An acid produces hydrogen ions HC in solution (an ion being a charged particle formed when atoms or molecules lose or gain electrons). Examples of acids include: sulphuric acid, H2SO4 , hydrochloric acid, HCl and nitric acid HNO3
A base is a substance that can neutralise an acid (i.e. remove the acidic properties of acids). An alkali is a soluble base. When in solution an alkali produces hydroxyl ions, OHÐ . Examples of alkalis include: sodium hydroxide, NaOH (i.e. caustic soda), calcium hydroxide, Ca(OH)2, ammonium hydroxide, NH4OH and potassium hydroxide, KOH (i.e. caustic potash).
A salt is the product of the neutralisation between an acid and a base, i.e.
Examples of salts include: sodium chloride, NaCl (i.e. common salt), potassium sulphate, K2SO4 , copper sulphate, CuSO4 and calcium carbonate, CaCO3 (i.e. limestone).
An indicator is a chemical substance, which when added to a solution, indicates the acidity or alkalinity of the solution by changing colour. Litmus is a simple two-colour indicator which turns red in the presence of acids and blue in the presence of alkalis. Two other examples of indicators are ethyl orange (red for acids, yellow for alkalis) and phenolphthalein (colourless for acids, pink for alkalis).
The pH scale (pH meaning ‘the potency of hydrogen’) represents, on a scale from 0 to 14, degrees of acidity and alkalinity. 0 is strongly acidic, 7 is neutral and 14 is strongly alkaline. Some average pH values include:
concentrated hydrochloric acid, HCl 1.0, lemon juice 3.0, milk 6.6, pure water 7.0, sea water 8.2, concentrated sodium hydroxide, NaOH 13.0
Acids have the following properties:
(i) Almost all acids react with carbonates and bicarbonates, (a carbonate being a compound containing carbon and oxygen — an example being sodium carbonate, i.e. washing soda)
(ii) Dilute acids have a sour taste; examples include citric acid (lemons), acetic acid (vinegar) and lactic acid (sour milk).
(iii) Acid solutions turn litmus paper red, methyl orange red and phenolph- thalein colourless, as mentioned above.
(iv) Most acids react with higher elements in the electrochemical series (see chapter 42) and hydrogen is released.
Alkalis have the following properties:
(i) Alkalis neutralise acids to form a salt and water only.
(ii) Alkalis have little effect on metals.
(iii) Alkalis turn litmus paper blue, methyl orange yellow and phenolphthalein pink, as mentioned above.
(iv) Alkalis are slippery when handled; strong alkalis are good solvents for certain oils and greases.
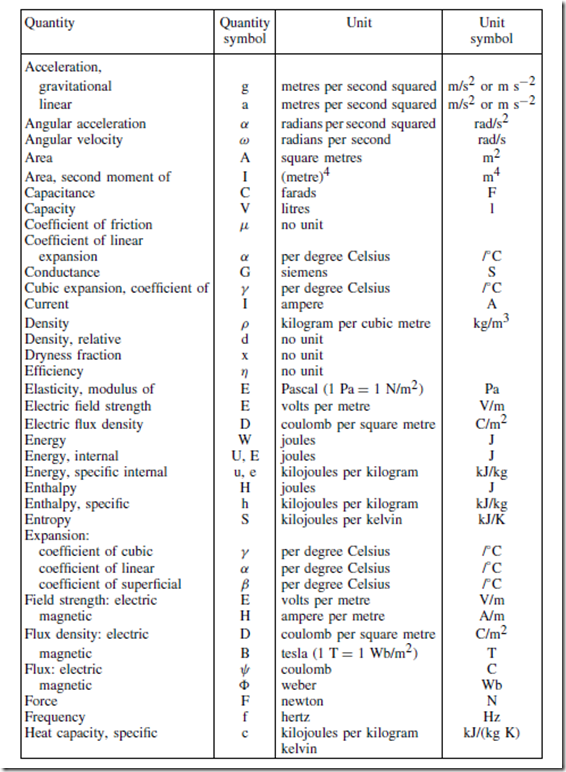
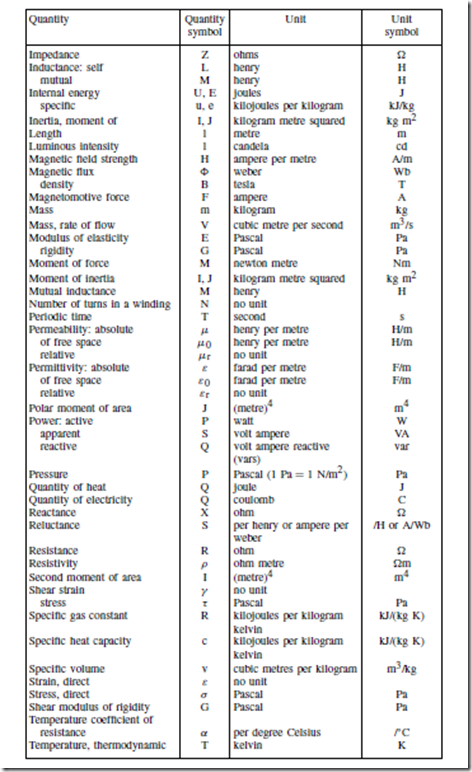
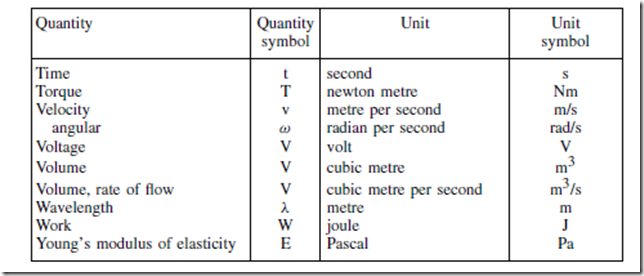
Standard Quantity Symbols and their Units