Elements
There are a very large number of different substances in existence, each sub- stance containing one or more of a number of basic materials called elements. ‘An element is a substance which cannot be separated into anything simpler by chemical means’. There are 92 naturally occurring elements and 13 others, which have been artificially produced.
Some examples of common elements with their symbols are: Hydrogen H, Helium He, Carbon C, Nitrogen N, Oxygen O, Sodium Na, Magnesium Mg, Aluminium Al, Silicon Si, Phosphorus P, Sulphur S, Potassium K, Calcium Ca, Iron Fe, Nickel Ni, Copper Cu, Zinc Zn, Silver Ag, Tin Sn, Gold Au, Mercury Hg, Lead Pb and Uranium U.
Atoms
Elements are made up of very small parts called atoms. ‘An atom is the smallest part of an element which can take part in a chemical change and which retains the properties of the element’.
Each of the elements has a unique type of atom.
In atomic theory, a model of an atom can be regarded as a miniature solar system. It consists of a central nucleus around which negatively charged particles called electrons orbit in certain fixed bands called shells. The nucleus contains positively charged particles called protons and particles having no electrical charge called neutrons.
An electron has a very small mass compared with protons and neutrons. An atom is electrically neutral, containing the same number of protons as electrons. The number of protons in an atom is called the atomic number of the element of which the atom is part. The arrangement of the elements in order of their atomic number is known as the periodic table.
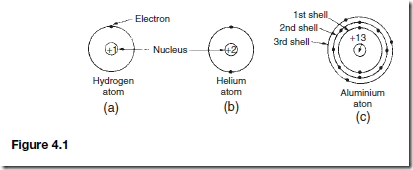
The simplest atom is hydrogen, which has 1 electron orbiting the nucleus and 1 proton in the nucleus. The atomic number of hydrogen is thus 1. The hydrogen atom is shown diagrammatically in Figure 4.1(a). Helium has 2 electrons orbiting the nucleus, both of then occupying the same shell at the same distance from the nucleus, as shown in Figure 4.1(b).
The first shell of an atom can have up to 2 electrons only, the second shell can have up to 8 electrons only and the third shell up to 18 electrons only. Thus an aluminium atom which has 13 electrons orbiting the nucleus is arranged as shown in Figure 1(c).
Molecules
When elements combine together, the atoms join to form a basic unit of new substance. This independent group of atoms bonded together is called a molecule. ‘A molecule is the smallest part of a substance which can have a separate stable existence’.
All molecules of the same substance are identical. Atoms and molecules are the basic building blocks from which matter is constructed.
Compounds
When elements combine chemically their atoms interlink to form molecules of a new substance called a compound. ‘A compound is a new substance containing two or more elements chemically combined so that their properties are changed’.
For example, the elements hydrogen and oxygen are quite unlike water, which is the compound they produce when chemically combined.
The components of a compound are in fixed proportion and are difficult to separate. Examples include:
(i) water H2O, where 1 molecule is formed by 2 hydrogen atoms combining with 1 oxygen atom,
(ii) carbon dioxide CO2 , where 1 molecule is formed by 1 carbon atom combining with 2 oxygen atoms,
(iii) sodium chloride NaCl (common salt), where 1 molecule is formed by 1 sodium atom combining with 1 chlorine atom, and
(iv) copper sulphate CuSO4 , where 1 molecule is formed by 1 copper atom, 1 sulphur atom and 4 oxygen atoms combining.
Mixtures
‘A mixture is a combination of substances which are not chemically joined together’. Mixtures have the same properties as their components. Also, the components of a mixture have no fixed proportion and are easy to separate. Examples include:
(i) oil and water
(ii) sugar and salt
(iii) air, which is a mixture of oxygen, nitrogen, carbon dioxide and other gases
(iv) iron and sulphur
(v) sand and water
Mortar is an example of a mixture — consisting of lime, sand and water.
Compounds can be distinguished from mixtures in the following ways:
(i) The properties of a compound are different to its constituent components whereas a mixture has the same properties as it constituent components.
(ii) The components of a compound are in fixed proportion whereas the components of a mixture have no fixed proportion.
(iii) The atoms of a compound are joined, whereas the atoms of a mixture are
free.
(iv) When a compound is formed, heat energy is produced or absorbed whereas when a mixture is formed little or no heat is produced or absorbed.
Solutions
‘A solution is a mixture in which other substances are dissolved’.
A solution is a mixture from which the two constituents may not be separated by leaving it to stand, or by filtration. For example, sugar dissolves in tea, salt dissolves in water and copper sulphate crystals dissolve in water leaving it a clear blue colour. The substance that is dissolved, which may be solid, liquid or gas, is called the solute, and the liquid in which it dissolves is called the solvent. Hence solvent Y solute = solution.
A solution has a clear appearance and remains unchanged with time.
Suspensions
‘A suspension is a mixture of a liquid and particles of a solid which do not dissolve in the liquid’.
The solid may be separated from the liquid by leaving the suspension to stand, or by filtration. Examples include:
(i) sand in water
(ii) chalk in water
(iii) petrol and water
Solubility
If a material dissolves in a liquid the material is said to be soluble. For example, sugar and salt are both soluble in water.
If, at a particular temperature, sugar is continually added to water and the mixture stirred there comes a point when no more sugar can dissolve. Such a solution is called saturated. ‘A solution is saturated if no more solute can be made to dissolve, with the temperature remaining constant’.
‘Solubility is a measure of the maximum amount of a solute which can be dissolved in 0.1 kg of a solvent, at a given temperature’. For example, the solubility of potassium chloride at 20°C is 34 g per 0.1 kg of water, or, its percentage solubility is 34%
(i) Solubility is dependent on temperature. When solids dissolve in liquids, as the temperature is increased, in most cases the amount of solid that will go into solution also increases. (More sugar is dissolved in a cup of hot tea than in the same amount of cold water.) There are exceptions to this, for the solubility of common salt in water remains almost constant and the solubility of calcium hydroxide decreases as the temperature increases.
(ii) Solubility is obtained more quickly when small particles of a substance are added to a liquid than when the same amount is added in large particles. For example, sugar lumps take longer to dissolve in tea than does granulated sugar.
(iii) A solid dissolves in a liquid more quickly if the mixture is stirred or shaken, i.e. solubility depends on the speed of agitation.
Crystals
A crystal is a regular, orderly arrangement of atoms or molecules forming a distinct pattern, i.e. an orderly packing of basic building blocks of matter. Most solids are crystalline in form and these include crystals such as common salt and sugar as well as the metals. Substances that are non-crystalline, are called amorphous, examples including glass and wood. Crystallisation is the process of isolating solids from solution in a crystalline form. This may be carried out by adding a solute to a solvent until saturation is reached, raising the temperature, adding more solute and repeating the process until a fairly strong solution is obtained, and then allowing the solution to cool, when crystals will separate. There are several examples of crystalline form that occur naturally, examples including graphite, quartz, diamond and common salt.
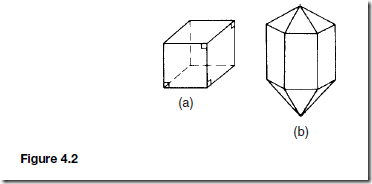
Crystals can vary in size but always have a regular geometric shape with flat faces, straight edges and having specific angles between the sides. Two common shapes of crystals are shown in Figure 4.2. The angles between the faces of the common salt crystal (Figure 4.2(a)) are always 90° and those of a quartz crystal (Figure 2(b)) are always 60° . A particular material always produces exactly the same shape of crystal.
Figure 4.3 shows a crystal lattice of sodium chloride. This is always a cubic shaped crystal being made up of 4 sodium atoms and 4 chlorine atoms. The sodium chloride crystals then join together as shown.
Metals
Metals are polycrystalline substances. This means that they are made up of a large number of crystals joined at the boundaries, the greater the number of boundaries the stronger the material.
Every metal, in the solid state, has its own crystal structure. To form an alloy, different metals are mixed when molten, since in the molten state they do not have a crystal lattice. The molten solution is then left to cool and solidify. The solid formed is a mixture of different crystals and an alloy is thus referred to as a solid solution. Examples include:
(i) brass, which is a combination of copper and zinc,
(ii) steel, which is mainly a combination of iron and carbon,
(iii) bronze, which is a combination of copper and tin.
Alloys are produced to enhance the properties of the metal, such as greater strength. For example, when a small proportion of nickel (say, 2% Ð 4%) is added to iron the strength of the material is greatly increased. By controlling the percentage of nickel added, materials having different specifications may be produced.
A metal may be hardened by heating it to a high temperature then cooling it very quickly. This produces a large number of crystals and therefore many boundaries. The greater the number of crystal boundaries, the stronger is the metal.
A metal is annealed by heating it to a high temperature and then allowing it to cool very slowly. This causes larger crystals, thus less boundaries and hence a softer metal.