Batteries
14–1 CHEMICAL ENERGY: A SOURCE OF EMF
In the preceding chapter we learned about the ionization and conductivity of salt or acid solutions called electrolytes. If two electrodes of different metals are immersed into such electrolyte, a chemical reaction between these parts will produce a small emf. Such an arrangement of parts is known as a cell. Cells are the building blocks of batteries. In other words, batteries are combinations of two or more cells for the attainment of higher voltages or currents.
Cells are classified, according to the nature of their chemical activity, as being primary or secondary. A primary cell obtains its energy by consuming one of its electrodes and cannot be restored when its active materials have been depleted. It is discarded at the end of its useful life. The common flashlight battery is an example of a primary cell.
By contrast, a secondary cell may be repetitively recharged after it has run down. The reason for this is that the chemical process within the cell is reversible. The lead-acid cells within a car battery are secondary cells.
The voltage derived from a cell depends solely on the type of materials used in the construction of the cell.
Figure 14–1 lists a number of metals in the order of the ease with which electrons escape into a water solution. Metals at the top of the list lose electrons readily, and those at the bottom of the list lose electrons less readily. The electromotive series list is quite different from a list arranged according to conductivity.
Although it is theoretically possible to make batteries from many combinations of materials, there are actually only a few practical combinations. Many combinations pro- mote undesirable chemical reactions that cause the active metal to corrode rapidly or to interfere with useful current production by building up resistance.
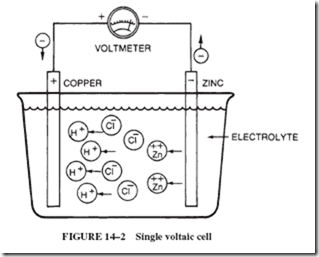
To gain some understanding of the process by which chemical interaction produces an emf, let us consider a simple, though impractical, cell made by placing a strip of zinc and a strip of copper into a weak solution of water and hydrochloric acid; see Figure 14–2. When a voltmeter is connected between the strips, an emf of approximately 1 volt is indicated.
From where does this energy come? The energy is due to the electrons of the zinc atoms and the hydrogen ions (H1) of the acid. All acids consist of positively charged H1 ions plus negative ions. The H1 ions attract electrons. Each zinc atom has two electrons
in its outer orbit that have sufficient energy to leave the zinc atom when they are subjected to the attraction of the H1 ions.
The transfer of electrons from atoms of zinc to H1 ions takes place when a piece of zinc is placed into an acid (whether or not another metal is present). When H1 ions take electrons from the zinc, they become neutral H atoms. Once the atoms are no longer positively charged, they are not attracted to the negative ions in the water. The H atoms then pair to form H2 molecules. These molecules are ordinary hydrogen gas that is visible as it bubbles away. The circle labeled Zn11 in Figure 14–3 represents
a zinc ion (a zinc atom that has lost two electrons). Zinc ions (Zn11) are attracted from the metal into the solution by the negative ions of the acid.
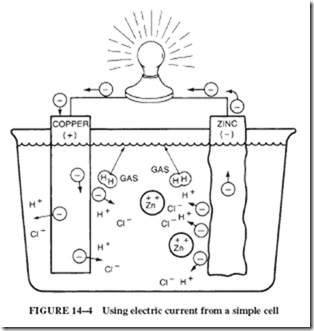
Electron transfer at the surface of the zinc bar does not result in a useful electric cur- rent. To obtain a useful current, a wire is connected between the zinc strip and the copper strip in the acid. The copper in the acid provides another source of electrons that can be attracted by the H1 ions, as shown in Figure 14–4. The electrons taken by the H1 ions at the copper surface are simultaneously replaced by electrons at the wire connection supplied by the zinc. The copper serves as a conductor and carries electrons away from the zinc and to the H1 ions.
Figure 14–4 shows a simple battery cell. However, this type of cell is impractical be- cause of the rapid, noncurrent-producing transfer of electrons at the surface of the zinc. A small strip of zinc gives away all of its energetic electrons and is distributed in the solution as zinc ions after just a few minutes of operation.
The Carbon-Zinc Cell (Leclanché Cell)
Georges Leclanché improved the basic battery cell by using a solution of water and ammonium chloride instead of hydrochloric acid. Ammonium chloride consists of NH41 and Cl2 ions. The ammonium ions, NH41, do not cause the zinc to be consumed as rapidly as the H1 ions of the HCl solution. Instead of a copper strip, Leclanché used a carbon rod surrounded by packed manganese dioxide. Manganese dioxide (MnO2) is a solid that does not dissolve in water. The manganese atoms in manganese dioxide have a strong positive charge and thus provide a strong attraction for electrons.
When a wire is connected between the zinc and the carbon rod, electrons flow from the zinc through the wire and carbon rod and are attracted to the positive manganese. At the same time, zinc ions are attracted into the ammonium chloride solution.
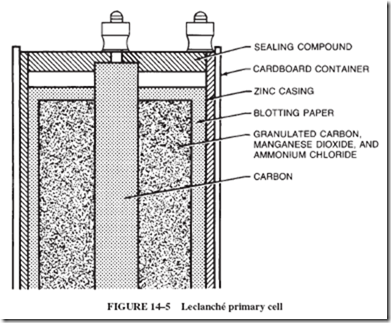
The combination of the carbon powder mixed with the manganese dioxide and the carbon rod acts as an inert conductor to carry electrons to the manganese dioxide in the cell. (The Mn1111 ions gain two electrons each to become Mn11 ions. However, these ions do not have an attraction for electrons that is strong enough for the electrons to be of any further use in the cell.) The typical dry cell used in flashlights and portable radios is a sealed Leclanché cell; see Figure 14–5. The inside of the cell is not dry but rather is a moist paste (when the cell is new). Zinc is often used as the outer container of a cell in which the container is the negative electrode. Dry cells cannot be recharged.
If a cell is to produce a large current in amperes, both the positive and negative terminals must have a large area of metal plate in contact with the electrolyte solution. This large area of contact makes it easy for electron transfers to occur. In other words, the resistance of the cell is low. A small penlight dry cell has just as much emf as the larger #6 dry cell, but the penlight cell cannot produce the same amount of current, because it has more internal resistance (this statement can be verified by Ohm’s law).
14–2 ANODES AND CATHODES
As mentioned in Chapter 13, the terminal by which electrons enter a device is called the cathode; the terminal where electrons leave the device is called the anode. The energy user in Figure 14–6 can be any device (a lamp; an electroplating bath) in
which electrons are forced onto the cathode by some other energy source. Electrons in the device are repelled from the cathode toward the anode, where they are attracted by an external energy source. The cathode of the energy user is negative (containing excess electrons) and the anode is positive. Electrons flow through the device from the cathode (negative) to the anode (positive).
For the energy producer in Figure 14–6, the anode is the metal that is rich in electrons (such as zinc). The anode supplies electrons to the external energy user. Electrons enter the cell at the cathode, since they are attracted to this location by a relatively positive electrode. The anode is negative; the cathode is positive. Thus, the energy in the cell pushes electrons out at the anode ( 2) and attracts them at the cathode ( 1).
The careful reader will observe that this conforms to our theory of current flow, which is that electrons move from negative to positive in the outer circuit (the load), but that in- side the source they move from positive to negative. This explains why the cathode of the source is labeled positive, while the anode is labeled negative.
14–3 PRIMARY CELLS
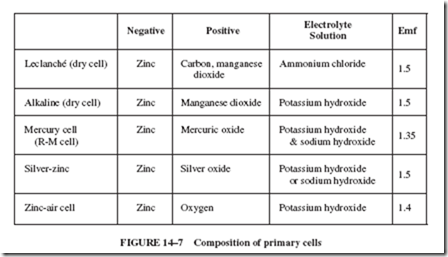
Each cell listed in Figure 14–7 is called a primary cell. In this type of cell, electron transfer is not readily reversible; that is, the dissolved zinc is not easily returned to its original metallic form. The dry cell cannot be reclaimed. A dry cell is the most commonly used primary cell. (Cells that can be recharged, such as those used in car batteries, are called secondary cells.)
Alkaline dry cells provide a greater current than carbon-zinc cells of equal size. Alkaline cells also provide a moderate current for a much longer time and can provide more power at low temperatures. These cells are useful in portable photoflash equipment, motion picture cameras, robot model planes, and for other applications where a better performance is worth the extra cost. A type of alkaline dry cell is rechargeable.
A mercury cell has a still higher energy potential for its size and weight when com- pared with the cells covered previously. In addition, the cost of this cell is greater. A mercury cell will maintain its 1.35-volt emf steadily for a long time. The mercury cells are used in handheld communication sets, hearing aids, and portable electronic equipment and appliances. This type of cell usually consists of a negative center terminal that is connected
to a zinc cylinder or pellet. The positive case is connected to a mixture of mercuric oxide and graphite. To avoid mercury contamination of soil and water, ordinary methods of trash disposal are not suitable for discarded mercury cells or other devices containing mercury.
The three types of dry cells just covered (Leclanché, alkaline, and mercury) are the most widely used dry cells. The silver-zinc cell, which is similar in construction to the mercury cell, maintains its emf throughout its life. Although the silver-zinc cell is expensive, it has a high ratio of energy to weight. This factor makes it useful in hearing aids, electric watches, and spacecrafts. Some silver cells are rechargeable.
In the zinc-air cell, the negative terminal leads to a porous zinc anode, which is soaked with the electrolyte solution. The cathode is a thin conductive plastic arrangement. The cell has enough wet resistance to prevent slow loss of moisture, yet it is porous enough to permit the entry of oxygen from the air. Finely divided platinum (or a silver alloy) on the cathode promotes an oxygen and water reaction that removes electrons from the cathode to form OH2 ions. Electrons are supplied to the load circuit by zinc atoms as they ionize. Some zinc-air cells are mechanically rechargeable; that is, the oxidized zinc anode (consisting mainly of potassium zincate) is removed, the cell is refilled with water, and a new porous zinc plate containing potassium hydroxide is inserted in the cell. Portable military communication equipment commonly uses zinc-air cells.
Many other primary cell combinations have been investigated, including magnesium- air, iron-air, lithium-nickel, and a 2-volt magnesium cell with magnesium bromide as the electrolyte and a cathode made of manganese dioxide.
The lithium battery, especially, needs to be mentioned here because it has gained wide acceptance in the electronics industry due to its unique properties. In low-current applications, such as the memory-retention circuits of computerized equipment, they often last for many years with virtually undiminished voltage output up to the last moments of life.
Actually, only very little lithium goes into the production of a cell; on average about 1⁄2 gram, which often comprises only about 5% of its total weight. Electrode material LiBF4 is dissolved into a nontoxic electrolyte material called gamma butyrolactone, which has a boiling point of 204°C, hence there is no outgassing during normal operation. In fact, some lithium batteries have operating temperatures from 240°F to 185°F.
Safety precautions must be taken if it becomes necessary to solder wires to the battery. The heat from the soldering iron can cause the battery to explode.
14–4 FUEL CELLS
All of the cells described to this point base their operation on the tendency of energetic electrons to transfer to a material that lacks electrons, such as a positively charged ion. During this process, the anode material itself is used up in the sense that it is converted to a useless low- energy form. The fuel cell also makes use of this same type of electron transfer but with the important difference that the material giving electrons and the material taking electrons are not contained within the cell. The solid electrodes of the cell are not consumed in the process.
The term fuel makes one think of something burning. During the burning (combustion) of any fuel, such as coal, oil, or gas, atoms of the fuel give electrons to oxygen in the air. The energy of the electrons immediately appears as heat. It is correct to think of the gradual consumption of the zinc in a flashlight battery as a slow oxidation (a slow burning process). Research over a number of years has produced very efficient fuel cells. The purpose of such cells is to control the electrons of inexpensive fuels and make them perform useful work as they leave the fuel atoms to join atoms of oxygen or some other electron taker. Fuel cells require high-purity fuel and inexpensive but reliable catalytic surfaces on which the essential reactions take place. Fuel cells also require auxiliary equipment such as gas containers and pressure controls. Research is under way to develop efficient, compact fuel cells for use in electric automobiles and tractors and as a source of power for a residence.
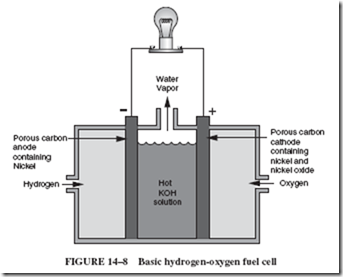
The hydrogen-oxygen fuel cell, as shown in Figure 14–8, has hollow, porous car- bon electrodes immersed in a potassium hydroxide solution. Hydrogen gas is pumped
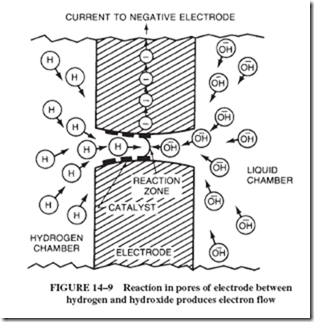
into one electrode and oxygen gas is pumped into the other. The porous carbon also contains certain metals or metal oxides called catalysts. (A catalyst promotes a chemical re- action.) In this case, the catalyst aids hydrogen molecules (which are pairs of hydrogen atoms) to separate into single atoms that can then combine with the negatively charged hydroxide ions (OH2) of the electrolyte. The combination of H and OH2 forms a molecule of water, H2O, with one electron left over. These surplus electrons are attracted to the electrode supplied by oxygen, as shown in Figure 14–9, where they take part in the reaction of oxygen 1 H2O 1 electrons to form hydroxide ions, OH2. Thus, hydroxide ions are re-formed at the cathode at the same rate as they are used up at the anode. The important resultant change is the conversion of hydrogen and oxygen to water.
The alkaline-hydrogen-oxygen fuel cell just discussed was one of the very early types introduced in the 1960s. There are other types of fuel cells today that offer different characteristics and advantages.
The Polymer Electrolyte Membrane (PEM) Fuel Cell
Polymer electrolyte membrane fuel cells are often called proton exchange membrane fuel cells. These cells offer high-power density combined with low weight and volume compared with other types of fuel cells. PEM fuel cells employ a solid polymer as the electrolyte. The electrodes are porous carbon combined with a platinum catalyst. They require only hydrogen, oxygen from the air, and water to operate. They do not need corrosive fluids like some other fuel cells. PEM fuel cells operate at a relatively low temperature, around 80°C (176°F).
Direct methanol fuel cells are powered by pure methanol mixed with steam. The methanol/steam mixture is fed directly to the fuel cell anode. These fuel cells have an advantage in that they do not have the fuel storage problems of cells that rely on pure hydrogen. Since methanol is a liquid, it is much easier to store. Methanol also has a higher energy density than hydrogen although it does not contain as much energy as gasoline or diesel fuel.
Phosphoric Acid Fuel Cells
Phosphoric acid fuel cells use liquid phosphoric acid as an electrolyte. The acid is contained in a Teflon-bonded silicon carbide matrix. The electrodes are composed of porous carbon containing a platinum catalyst. This cell is considered the first generation of modern fuel cells and has been used commercially for stationary power generation and to power large vehicles such as city buses.
Molten Carbonate Fuel Cells
Molten carbonate fuel cells are being developed for natural gas and coal fired power plants. These fuel cells operate at very high temperatures, typically 650°C (1200°F) and above. The high operating temperature has an advantage in that non-precious metals can be used as a catalyst in the anode and cathode electrodes, resulting in much lower cost. Molten carbonate fuel cells can reach efficiencies as high as 60%.
Solid Oxide Fuel Cells
Solid oxide fuel cells use a hard non-porous ceramic compound as the electrolyte. Because the electrolyte is solid, the fuel cell does not have to be constructed in the plate like configuration typical of other types of fuel cells. These fuel cells operate at very high temperatures, 1,000°C (1,830°F). The high operating temperature has an advantage in that non-precious metals can be used as a catalyst in the electrodes. Also, the high operating temperature of the solid oxide fuel cell permits reforming of fuels internally, enabling use of a variety of fuels, which helps reduce the cost of adding a reformer to the system. Solid oxide fuel cells have an efficiency of 50% to 60%.