Conduction in Liquids and Gases
13–1 THE IONIZATION PROCESS
In solid conductors, the transfer of electrical charges is made by the movement of electrons. In liquids and gases, the transfer of electrical charges depends on particles
called ions. Ions were defined in Chapter 1 as atoms that have become electrically unbalanced by virtue of having lost or gained electrons.
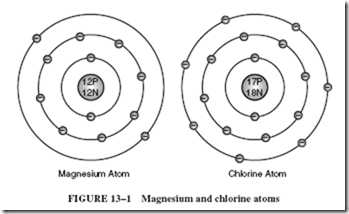
To briefly review this concept, consider the model of a magnesium atom alongside a chlorine atom, as shown in Figure 13–1.
You should recall that magnesium is classified as a metal because it has only two valence electrons. By contrast, chlorine gas has seven valence electrons and is, therefore, classified as a nonconductor.
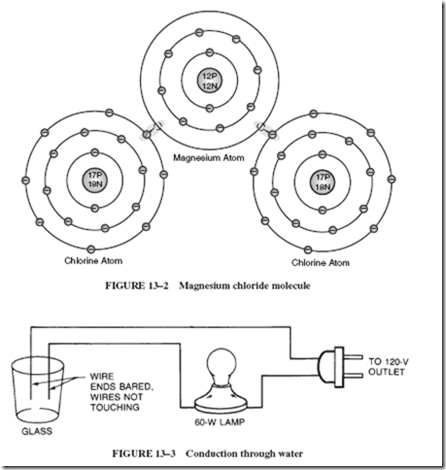
When a piece of magnesium is heated in the presence of chlorine gas, the two substances will chemically interact to form a new compound: a metallic salt called magnesium chloride. During this chemical process, each magnesium atom gives up its two valence electrons, which are then transferred to two neighboring atoms, as shown in Figure 13–2. These atoms are no longer known as atoms, since they now have been electrically charged. After an originally neutral atom becomes electrically charged, it is called an ion.
The magnesium atom has lost electrons (negative charges) and is now positively charged. Thus, it is called a positive ion. Likewise, the chlorine atoms have gained additional electrons and have been transformed into negative ions. Notice in Figure 13–2 that each ion has eight electrons in its outer shell.
This newly formed substance, magnesium chloride, is similar to table salt. The chemical name for table salt is sodium chloride; that is, the elements that make up table salt are a metal, sodium, and a nonmetal, chlorine.
The conducting ability of a solution of salt in water can be demonstrated with the equipment shown in Figure 13–3. First, fill the glass with kerosene, and then connect the circuit to the 120-volt line. Nothing happens to the lamp as a result of this step. Now add some table salt or magnesium chloride to the kerosene. The neutral molecules of the kerosene do not attract the undissolved charged ions of the salt. Again, there is no current through the lamp.
Fill another glass with pure distilled water (if available) and connect the circuit. Note that nothing happens. If water from the faucet is used in the glass, the lamp may light very dimly. When salt is added to the water and is stirred to dissolve, the lamp brightens. What causes this increased conductivity when magnesium chloride or sodium
chloride is dissolved in water? The dissolved substance does not retain its molecular structure but breaks up into its ion particles, which are free to move through the liquid.
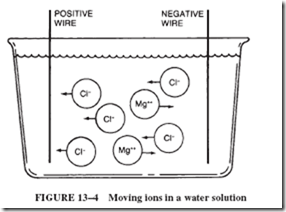
Figure 13–4 shows a water solution consisting of negative chlorine ions and positive magnesium ions. When two electrically charged wires are submerged into the solution, a two-way movement of ions takes place. The negative chlorine ions are being attracted by the positive wire, and the positive magnesium ions are pulled toward the negative wire. When the ions reach their respective wires, the resulting action depends on the kinds of ions and the type of wire used.
In this manner, water solutions of acids, bases, and metallic salts can conduct large amounts of current. Such solutions are known as electrolytes.
 The following points summarize the information presented so far:
The following points summarize the information presented so far:
• Metals and nonmetals unite to form compounds by transferring electrons from the metallic atoms to the nonmetallic atoms.
• As a result of the electron transfer, the compound consists of positively charged metallic
ions and negatively charged nonmetallic ions.
• Some compounds dissolve in water and dissociate; that is, the ions become separated and move freely. Ions are much larger and heavier than electrons.
• Conduction in metals consists of the movement of free (negative) electrons.
• Conduction in solutions, known as electrolytes, consists of the movement of free positive and negative ions in opposite directions.
13–2 USEFUL CHEMICAL COMPOUNDS
The ions covered so far are formed by the electrical charging (either positive or negative) of a single atom. More complicated ions, however, do exist. These ions consist of charged groups of atoms. The following four compounds, each of which is useful in electrical processes, are formed, in part, of these larger ions.
Sulfuric Acid. This compound is used as the electrolyte in automobile batteries. The chemical formula for sulfuric acid is H2SO4. In other words, one molecule of the acid contains two atoms of hydrogen, one atom of sulfur, and four atoms of oxygen. When mixed with water, each molecule can separate into the ions shown: H1 H1 SO422. The 1 sign on the H indicates a hydrogen ion (a hydrogen atom that has lost its one electron). The is called a sulfate ion and consists of one sulfur atom, four oxygen atoms, and two extra electrons. (Notice that an ion can be either one electrically charged atom or a group of such atoms.) The two electrons are necessary to hold the SO4 group together. The electrons are part of an electron-sharing arrangement that keeps the electron rings of the sulfur and oxygen atoms tied together.
Ammonium Chloride. The electrolyte in a dry cell is a solution of ammonium chloride dissolved in water. Pure ammonium chloride is a white solid. The formula for ammonium
chloride is NH4Cl. Ammonium chloride separates into two ions when it dissolves: NH41 and Cl2. The symbol NH41 means that one nitrogen atom and four hydrogen atoms are grouped together with the loss of one electron. The electron lost by the NH4 group is taken by the chlorine atom to form a negatively charged chlorine ion. The NH41 group is called an ammonium ion.
Cuprous Cyanide. The commercial process of electroplating copper on iron uses a poi- sonous solid called cuprous cyanide as the plating solution. The formula for cuprous cyanide is CuCN. In solution, the compound separates into the ions Cu1 and CN2. Cu is the symbol for copper. Cu1 represents the cuprous ion (a copper atom that has lost its outer- most electron). CN2 is one atom of carbon and one atom of nitrogen held together with the help of one electron taken from the copper atom. CN2 is called the cyanide ion.
Copper Sulfate. Some copper-plating processes use a copper compound called copper sulfate, CuSO4. Copper sulfate is a solid in the form of blue crystals. When copper sulfate dissolves in water, it forms Cu11 and SO422 ions. Under some conditions, a copper atom can lose two of its electrons to form an ion called a cupric ion. SO422 is the same sulfate ion that appears in sulfuric acid.
13–3 ELECTROPLATING AND ELECTROLYSIS
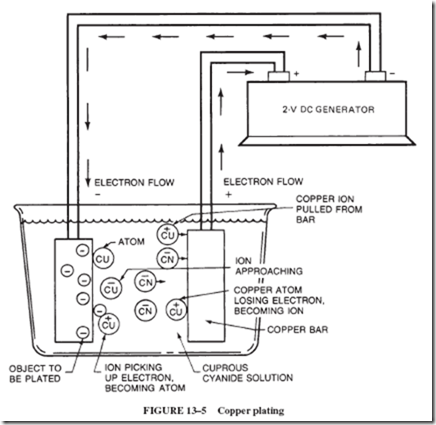
To illustrate the electrical process that occurs in the plating of metals, let us examine the plating of an object with copper from a cyanide solution. The object to be plated, Figure 13–5, must be an electrical conductor. It is connected to the negative terminal of a battery or DC generator and is covered by the cuprous cyanide solution. The positive terminal of the supply is connected to a copper bar, which is also covered by the solution. When the circuit is complete, positively charged copper ions in the liquid move toward the object to be plated. When the copper ions touch the negatively charged object, the ions pick up electrons and become neutral atoms of copper. As the ions become atoms at the surface of the negatively charged object, they form a copper coating over the object. As long as the generator pushes electrons onto the object to be plated so that its negative charge is maintained, a copper coating of increasing thickness will be deposited on the object.
When the cyanide ions hit the positive copper bar, copper atoms on the surface of the bar lose electrons and become ions. In other words, the negative CN ion causes a copper atom to lose an electron and become a positive ion. The copper ion is attracted into the solution by the negative ions present in the solution. The electrons lost by the copper atoms drift toward the generator. Just as many copper ions are liberated from the copper bar as are de- posited on the object to be plated; therefore, the solution stays at a constant strength.
In any electroplating process, the solution must contain the ions of the metal that is to form the coating. Since metal ions are all positively charged, the object to be plated is connected to the negative wire. The positive terminal normally is made of the same metal that is to form the coating. Plating solutions also contain ingredients other than the dissolved metal compound. These other ingredients are added to prevent corrosion of the object to be plated, to prevent poisonous fumes, and to aid in forming a smooth coating.
The purification of metals can be accomplished through a large-scale plating process. Almost all commercial copper is refined by plating in a copper sulfate solution.
Pure copper is deposited on the negative plate. Impurities from the crude copper on the positive terminal either stay in the solution or never leave the copper bar at all. Impure copper has a high resistance. Copper wire manufacturers require electrolytically purified copper in the production of low-resistance conductor wire. Zinc and other metals are purified in a similar fashion.
The term electrolysis refers to the process of separating elements by the use of electrical energy. The commercial production of metallic and nonmetallic elements is often a matter of separating the element from others with which it is chemically combined. In 1885, aluminum was a rare and precious metal because it was difficult to separate from its abundant compound, aluminum oxide. Aluminum metal became inexpensive when a process was discovered for separating the aluminum from the oxygen. The extraction of aluminum is an electrical process in which electrons are removed from oxygen ions and are returned to aluminum ions. Magnesium metal is extracted from magnesium chloride (from seawater) by electrically separating the magnesium ions from the chlorine ions.
One process that has an important application in batteries is the electrolysis of water containing sulfuric acid. Hydrogen and oxygen gases are produced in this process; however, the commercial production of large amounts of these gases is achieved more economically by other processes.
Electrolysis of Sulfuric Acid in Water
Two new terms are shown in Figure 13–6: the cathode is the terminal or electrode where electrons enter the cell, and the anode is the electrode that carries electrons away from the cell.
Assume that the cathode and anode are made of materials that are not affected by the sulfuric acid solution or by the hydrogen or oxygen released in the process. When hydrogen ions touch the cathode, they pick up electrons from it and become neutral atoms. These atoms of hydrogen form hydrogen gas, which escapes from the solution as bubbles. When the sulfate ions approach the anode, they cause the water molecules to break up. The positive anode requires electrons, and the water molecules part with electrons more readily than the SO422 ions. Therefore, the removal of two electrons from the H2O molecule leaves two H1 ions and one oxygen atom. The oxygen atoms bubble away as gas at the anode, and the H1 ions stay in the solution. For each pair of hydrogen ions formed at the anode, a pair of hydrogen ions is discharged as gas at the cathode. The amount of acid in the solution remains constant, and the water is gradually consumed.
If lead plates are used for the cathode and anode, then not all of the oxygen bubbles away. A portion of the oxygen combines with the lead anode to form lead dioxide. The hydrogen does not affect the cathode, which remains as pure lead.
Applications of plates of lead and lead dioxide in a solution of sulfuric acid relates to the working principles of storage batteries. More detailed information about this principle is offered in Section 14–5.
Electrolytic Corrosion
Corrosion is the rusting or wearing away of a metal by the formation of a chemical compound of the metal. In some instances, the corrosion process is desirable. For example, in copper plating, the copper anode dissolves and in that way maintains the necessary copper ions in the plating solution. An example of an undesirable type of corrosion is shown when there is an electrical current through the earth that can follow buried water pipes for a portion of its path. At any points where the buried metal is positive (anodic) as compared to the earth, the attack on the pipe by negative ions converts the metal atoms into metal ions; in other words, iron pipe is converted into iron rust.