Refrigerant leak detection
A leak test is a vital operation after the installation or service of refrigeration equipment. An efficient leak test will prevent expensive call-backs and losses.
Shortage of refrigerant in a system results in reduced plant capacity and thus in longer running times of the equipment, which could prove costly to the customer over a prolonged period. It can also be the cause of a major breakdown of a compressor due to inadequate lubrication and cooling, as explained in Chapter 10.
A system must be operating with a full charge of refrigerant to enable a commissioning engineer to obtain the design conditions by the setting of various controls (see Chapter 16).
Dye systems
Some plants may be charged at the time of installation with a refrigerant which contains a coloured dye to provide a visual indication of leakage. Systems using refrigerant containing a dye must always be liquid charged. This is most important when adding refrigerant to a system after a considerable loss due to leakage.
The dye tends to separate from the refrigerant in the cylinder and is heavier than the liquid refrigerant. Therefore it is advisable to agitate the liquid when charging or adding refrigerant by placing the cylinder in a horizontal position and rocking it gently.
Refrigerant charging procedures are dealt with in Chapter 4.
Test pressures
During normal service operations a leak test does not entail a prior evacuation of the system unless the system is contaminated or the refrigerant has been completely discharged. It is essential however, that a minimum of 30 psig (pounds per square inch gauge) or 2 bar exists in the system when testing for
leaks. If no leaks are found then the system should be tested again at operating pressures.
A pressure leak test is more satisfactory and will be dealt with later.
Methods of testing
Note: Refer to Chapter 16 on refrigerants and revised procedures for leak detection, evacuation during commissioning.
Bubble test
The most common and inexpensive test is the bubble test. A water/soap solu- tion is simply brushed around a joint or component which is suspected of leaking, or sprayed on with an aerosol. It is recommended that proprietary rather than made-up solutions are used, as they are more viscous and the bubbles are stronger and longer lasting. Ordinary soap bubbles are weaker and are normally short lived.
The disadvantage of this method is that a large leak can blow through the solution and then no bubbles will appear, although in most such cases the leak will be audible.
Halide torch
This consists of a small burner assembly mounted on top of a container of gas, for example propane. The burner comprises a hand valve and a venturi or mixing chamber with an attachment for the exploring tube. Above the orifice of the burner there is a copper ring, a strip or a tube through which the flame passes when the torch is ignited.
When the torch is lit the air will be drawn into the venturi via the exploring tube, and the flame will burn slightly blue or colourless. When a trace of halogen refrigerant (R11, R12, R22, R500, R502 etc.) mixes with the air, the flame will immediately change colour as the refrigerant vapour contacts the hot copper ring or tube. The colour will range from green for a small leak to dark blue or purple for a large leak. When the refrigerant burns off, a toxic atmosphere will be created.
To test for leaks the exploring tube is passed around the suspected area slowly, and for effectiveness the plant should be stopped.
Electronic leak detector
This is the most sensitive type of leak detector, and many designs are avail- able. Some respond to an ion source, and others to a change of temperature (thermistor); the dielectric type is based on the conductivity of different gases.
These instruments are dry cell battery operated. When used the sensor or sensing tip should be inspected for cleanliness; tips should always be kept free from dirt and lint. Filters should be changed regularly, because a contaminated filter will cause the instrument to respond as if a leak was detected.
Normally the instruments will respond to atmospheric air by giving out an audio signal (bleep) at approximately one bleep per second. When the halogenated refrigerant contacts the sensor the signal will accelerate, depending upon the degree of vapour leaking; a large leak could produce a continuous signal or oscillation.
One disadvantage is that because of the sensitivity of the instrument it will respond to minute volumes of refrigerant vapour, and sometimes it will prevent the actual pin-pointing of a leak. It is also responsive to expanded foam insulants, thereby making somewhat difficult the detection of leaks on pipework passing through coldroom walls and parts of domestic appliance systems.
When using a detector the amount of air movement must be reduced to a minimum, i.e. all fans should be switched off and draughts excluded. The sensing tip should be applied below a joint because refrigerant vapour is heavier than air, and then moved slowly around the area.
Nessler’s reagent
This is a chemical solution used to detect leaks on water cooled systems charged with ammonia, which has an affinity for water. The solution is added to the recirculated water. If ammonia is present in the water, the solution will react to the nitrates contained therein to change the colour of the water to brown.
Sulphur candle
This takes the form of a small candle or taper which, when ignited, will smoulder and give off sulphur fumes. It is also used to detect leaks on pipework and components in ammonia systems. When it is passed around
a joint suspected of leaking, the ammonia fumes will mix with the sulphur fumes to produce a white vapour.
Both ammonia and sulphur fumes are toxic. Thus adequate ventilation must be provided, and precautions must be taken not to inhale the fumes. Ammonia leaks are easily detected by its pronounced odour at 3 to 5 parts per million. At approximately 15 ppm the vapour is very toxic, and at 30 ppm a suitable respirator will be required. Ammonia is lethal at 5000 ppm, and the maximum exposure time to atmospheres of 50 ppm is 5 minutes.
Ammonia becomes flammable at 150 000 to 270 000 ppm.
Halogenated refrigerants
These are compounds which contain one or more of the halogens, such as fluorine, chlorine, iodine and bromine. When they are exposed to a naked flame they will burn and produce a pungent odour, which can be injurious to the human respiratory system.
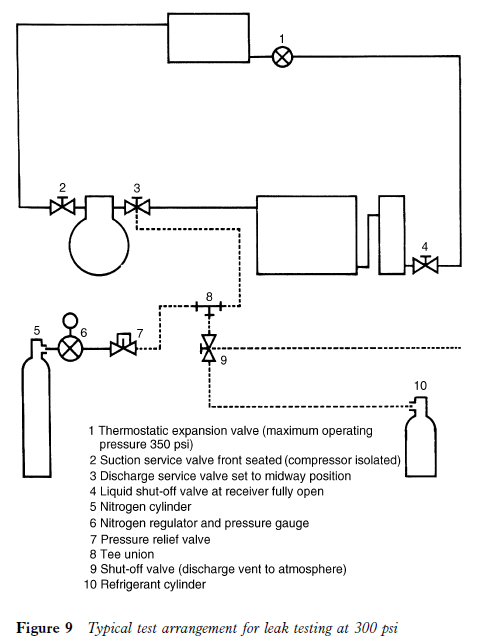
Pressure leak testing
This is carried out on new system installations or when a plant has been discharged of refrigerant prior to repair. It involves the use of oxygen-free nitrogen (OFN) which is a high pressure gas. This is used to obtain a higher pressure than that of the refrigerant in normal ambient temperatures. This pressure should be controlled and in excess of that under which the system is expected to operate with a normal operating charge of refrigerant. This could be as high as 500 psig (33 bar) in some instances.
Sometimes it is the practice to test the system pipework only in this manner before connecting to the condensing unit. If the condensing unit is new it would have already undergone very stringent tests to pressure vessel standards by the manufacturer.
When an installation is completed and a pressure test is to be carried out it is most important to ensure that the compressor is isolated, irrespective of design, before pressurising the system. The suction service valve should be front seated to avoid any damage to the compressor valves. This will also prevent rupture of the crankshaft seals in open type and semi-hermetic motor compressors.
All pressure controls must be disconnected or by-passed. If the expansion valve is not capable of withstanding the test pressure then it too must be
removed or by-passed. The bellows or diaphragm of an expansion valve has a maximum operating pressure which must not be exceeded.
The OFN cylinder must be fitted with an approved regulator to control the test pressure. For safety reasons a pressure relief valve preset to the test pressure is recommended.
When the system is pressurized, that pressure should be recorded and the plant left for a reasonable period. Just how long that period should be is debatable. It could take a considerable time for a noticeable drop in the nitrogen pressure to become evident when a system has a small leak. The time allocated by some installers and service outlets varies, and with large installations periods of days under pressure is not uncommon.
With the time element being an important factor a ‘bubble test’ may be permissible but this is not accepted by all suppliers of refrigerants.
British Standard 4434 1995 states that only an inert gas may be used for pressure testing. R22 or any other refrigerant is not to be used as a ‘trace’ pressure test. The ‘trace’ is no longer regarded as good practice. (For reference, the trace method of leak detection involved charging a system with a small amount of its operating refrigerant and boosting the pressure within the system with nitrogen. The leak could then be detected using a halide torch. Another method of leak detection is to draw a vacuum on the complete system but again the waiting period will be necessary to see if the vacuum is held. The disadvantage of drawing a vaccum would be the ingress of air which contains moisture if a slight leak were to break the vacuum.
Handling refrigerants: safety precautions
Although the common refrigerants (R12, R22, R502 etc.) are not considered hazardous, it must be remembered that all refrigerants are heavier than air and will replace air in a confined space very quickly. This can be dangerous; if the air does not contain at least 19 per cent oxygen, loss of consciousness may result.
When testing for leaks, always ensure that the area is well ventilated if at all possible. Always stand to one side of the detector in case there is a sudden violent discharge from the suspected pipework.
The following precautions should be taken:
1 Wear goggles, gloves and overalls at all times to protect eyes and to prevent direct contact of refrigerant with the skin, which can cause burns. This applies especially when charging or discharging refrigerant.
2 Make sure that the service cylinder is not overfilled.
 3 Do not expose cylinders to direct sunlight, radiated heat or convected heat from appliances.
3 Do not expose cylinders to direct sunlight, radiated heat or convected heat from appliances.
4 Avoid discharge near naked flames or flame producing appliances.
5 Avoid direct contact with refrigerant/oil solutions from hermetic systems (motor burn-out), which can be very acidic.
6 Always vapour charge a system from the low side to avoid possible damage to compressor valves.
7 Always check that the refrigerant is correct for the system being charged.
8 Whenever possible ensure that the working area is well ventilated. If ammonia is being used, ensure that a respirator or some form of breathing apparatus is at hand.
