2.5 Equations of State for a Nonideal Gas
Nonideal-gas behavior occurs when the pressure is relatively high (> 2 MPa for many gases) or when the temperature is near the saturation temperature. There are no acceptable criteria that can be used to determine if the ideal-gas equation can be used or if nonideal-gas equations must be used. Usually a problem is stated in such a way that it is obvious that nonideal gas effects must be included; otherwise a problem is solved assuming an ideal gas.
The van der Waals equation of state accounts for the volume occupied by the gas molecules and for the attractive forces between molecules. It is ![]()
where the constants a and b are related to the critical-point data of Table B.3 by
These constants are also presented in Table B.7 to simplify calculations.
An improved equation is the Redlich Kwong equation of state: 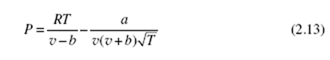
where the constants are also included in Table B.7 and are given by 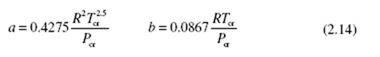
EXAMPLE 2.6
Calculate the pressure of steam at a temperature of 500°C and a density of 24 kg/m3
using (a) the ideal-gas equation, (b) the van der Waals equation, (c) the Redlich Kwong equation, (d ) the compressibility factor, and (e) the steam table.
Solution
(a) Using the ideal-gas equation,
P = pRT = 24 × 0.462 × 773 = 8570 kPa
where the gas constant for steam is found in Table B.2.
(b) Using values for a and b from Table B.7, the van der Waals equation provides 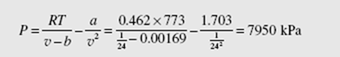
(c) Using values for a and b from Table B.7, the Redlich-Kwong equation gives 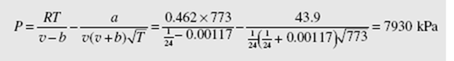
(d) The compressibility factor is found from the generalized compressibility chart of App. G. To use the chart we must know the reduced temperature and pressure (using Table B.3): 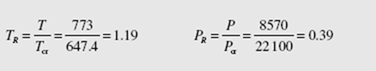
where we have used the ideal-gas pressure from part (a). Using the compressibility chart (it is fairly insensitive to the precise values of TRand PRso estimates of these values are quite acceptable) and Eq. (2.9), we find 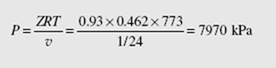
(e) The steam table provides the best value for the pressure. Using T =
500°C and v = 1/24 = 0.0417 m3/kg, we find P = 8000 kPa.
Note that the ideal-gas law has an error of 7.1 percent, and the error of each of the other three equations is less than 1 percent.
Quiz No. 1
1. The phase change from a vapor to a liquid is referred to as
(A) vaporization
(B) condensation
(C) sublimation
(D) melting
2. The volume occupied by 4 kg of 200°C steam at a quality of 80 percent is
nearest
(A) 0.004 m3
(B) 0.104 m3
(C) 0.4 m3
(D) 4.1 m3
3. For a specific volume of 0.2 m3/kg, the quality of steam, if the absolute pressure is 630 kPa, is nearest
(A) 0.44
(B) 0.50
(C) 0.59
(D) 0.66
4. Saturated liquid water occupies a volume of 1.2 m3. Heat is added until it is completely vaporized. If the pressure is held constant at 600 kPa, the final volume is nearest
(A) 344 m3
(B) 290 m3
(C) 203 m3
(D) 198 m3
5. Saturated steam is heated in a rigid tank from 70 to 800°C. P2 is nearest
(A) 100 kPa
(B) 200 kPa
(C) 300 kPa
(D) 400 kPa
6. Determine the final volume if 3 kg of water is heated at a constant pressure until the quality is 60 percent. The pressure is 270 kPa.
(A) 1.07 m3
(B) 1.24 m3
(C) 1.39 m3
(D) 2.93 m3
7. A vertical circular cylinder holds a height of 1 cm of liquid water and 100 cm of vapor. If P = 200 kPa, the quality is nearest
(A) 0.01
(B) 0.1
(C) 0.4
(D) 0.8
8. A rigid vessel with a volume of 10 m3 contains a water-vapor mixture at 400 kPa at 60 percent quality. The pressure is lowered to 300 kPa by cooling the vessel. Find m
at state 2.
(A) 16.5 kg
(B) 19.5 kg
(C) 23.8 kg
(D) 29.2 kg
9. The mass of air in a tire with a volume of 0.2 m3 with a gage pressure of 280 kPa at 25°C is nearest
(A) 7.8 kg
(B) 0.889 kg
(C) 0.732 kg
(D) 0.655 kg
10. Estimate the difference in density between the inside and outside of a house in the winter when P = 100 kPa, Tinside = 20°C, and Toutside = −20°C. (Would
you expect any tendency for air exchange due to this difference?)
(A) 0.19 kg/m3
(B) 0.17 kg/m3
(C) 0.15 kg/m3
(D) 0.09 kg/m3
11. Estimate the pressure of nitrogen at a temperature of 220 K and a specific volume of 0.04 m3/kg using the van der Waals equation.
(A) 1630 kPa
(B) 1600 kPa
(C) 1580 kPa
(D) 1540 kPa
12. Ten kilograms of 400°C steam are contained in a 734-L tank. Calculate the
error in the pressure if it is calculated using the ideal-gas equation (rather
than the more accurate steam tables).
(A) 1%
(B) 4%
(C) 6%
(D) 18%
Quiz No. 2
1. The point that connects the saturated-liquid line to the saturated-vapor line is called the
(A) triple point
(B) critical point
(C) superheated point
(D) compressed liquid point
2. Estimate the volume occupied by 10 kg of water at 200°C and 2 MPa.
(A) 0.099 m3
(B) 0.016 m3
(C) 11.6 L
(D) 11.8 L
3. The specific volume of water at 200 °C and 80 percent quality is nearest
(A) 0.06 m3/kg
(B) 0.08 m3/kg
(C) 0.1 m3/kg
(D) 0.12 m3/kg
4. Calculate the specific volume of water at 221°C if the quality is 85 percent.
(A) 0.072 m3/kg
(B) 0.074 m3/kg
(C) 0.76 m3/kg
(D) 0.78 m3/kg
5. Five kilograms of steam occupy a volume of 10 m3. If the temperature is 86°C, the quality and the pressure are nearest
(A) 0.76, 60 kPa
(B) 0.69, 68 kPa
(C) 0.71, 63 kPa
(D) 0.72, 61 kPa
6. Water at an initial quality of 50 percent is heated at constant volume from 100 kPa to 200°C. What is P2?
(A) 162 kPa
(B) 260 kPa
(C) 370 kPa
(D) 480 kPa
7. Find the quality of steam at 120°C if the vapor occupies 1200 L and the
liquid occupies 2 L.
(A) 42%
(B) 28%
(C) 18%
(D) 4%
8. Two kilograms of steam are contained in a piston-cylinder arrangement.
The 20-mm-diameter 48-kg piston is allowed to rise with no friction until the temperature reaches 260°C. The final volume is nearest
(A) 0.13 m3
(B) 0.29 m3
(C) 0.34 m3
(D) 0.39 m3
9. Estimate the temperature of 2 kg of air contained in a 40 L volume at 2 MPa.
(A) 125 K
(B) 140 K
(C) 155 K
(D) 175 K
10. The gage pressure reading on an automobile tire is 240 kPa when the tire temperature is −30°C. The automobile is driven to a warmer climate and the tire temperature increases to 65°C. Estimate the gage pressure in
the tire making reasonable assumptions.
(A) 480 kPa
(B) 370 kPa
(C) 320 kPa
(D) 280 kPa
11. Estimate the pressure of nitrogen at a temperature of 220 K and a specific volume of 0.04 m3/kg using the compressibility factor.
(A) 1630 kPa
(B) 1620 kPa
(C) 1580 kPa
(D) 1600 kPa
12. Ten kilograms of 100°C steam are contained in a 17 000-L tank. Calculate
the error in the pressure using the ideal-gas equation.
(A) 1%
(B) 4%
(C) 6%
(D) 18%
