Galileo’s air themometer : Introduction to Gas laws
The thermal expansion of air was first put to practical use in 1592 by Galileo Galilei, who used it as a crude method of indicating temperature changes.
Galileo’s air thermometer consists of a glass bulb about the size of a hen’s egg attached to a long, thin tube which dipped into a vessel of water.
Amodel of this thermometer may easily be made by fitting a glass flask with a rubber bung and a length of glass quill tubing.
This is set up vertically with the end of the tube below the surface of some water in a vessel. On placing a warm hand over the flask, the air inside becomes heated and expands.
Bubbles are seen to emerge from the lower end of the tube and, when the hand is removed, the air in the flask contracts and the water rises in the tube.
Subsequent changes of temperature will cause the water either to rise or fall in the tube.
Galileo attached a scale to his instrument and marked it in degrees, but he made no attempt to standarize the scale by the use of fixed temperature points.
A serious fault of Galileo’s air themometer
An air thermometer of this type has a very serious fault. Its readings are affected by changes in atmospheric pressure as well as by temperature. This defect was pointed out in 1665 by Robert Boyle, who had recently made a study of the way in which the volume of a gas alters with pressure when its temperature is kept constant.
Experiments to measure the thermal expansion of a gas are, therefore, bound to be complicated by the fact that the volume can be altered by a change in pressure as well as by a change in temperature. The difficulty does not rise in the case of solids or liquids, as these are very much less compressible
In order to make a full study of the behaviour of a gas as regards volume, temperature and pressure, three separate experiments have to be carried out to investigate respectively :
(1) The relation between volume and pressure at constant temperature (Boyle’s law);
(2) the relation between volume and temperature at constant pressure (Charles’s law);
(3) the relation betwen pressure and temperature at constant volume(Pressure law).
Robert Boyle’s work on air : Boyle’s law
 |
| Boyle’s experiment |
The law relating the volume and pressure of a fixed mass of air kept at constant temperature was first investigated experimentally by Robert Boyle about the middle of the seventeenth century.
In the first instance he used a glass tube in the form of a letter J having its shorter arm closed.
Mercury was added so as to trap air in the short arm and the quantity of air adjusted so that the levels were the same on both sides. More mercury was then poured in until the difference in levels was equal to the barometric height ( about 760 mmHg).
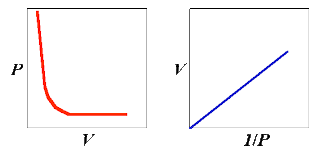
Having thus doubled the pressure, it was noticed that the volume of the air had been halved. This kind of relationship is called inverse proportion.
Expressed mathematically, volume µ1/pressure
or V∝1/p
It follows that V = constant × 1/p
Altogether, Boyle took about twenty-five pairs of readings for different pressures and volumes and found that they were in very close agreement with the above equation.
The pressure was found by adding the difference in the mercury levels to the barometric height. The volume was taken as being proportional to the length of the air column in the closed tube.
The J-tube, of course, gave only readings for pressures above atmospheric.
For lower pressures he used a straight tube closed at its upper end and dipping into a long jar of mercury. This was raised to various heights out of the mercury and the pressure found by subtracting the difference in mercury levels, h in mm, from the barometric height.
Later experiments showed that all the so-called permanent gases behave in the same way as air, and so the result may be expressed as a universal law.
Boyle’s Law
The volume of a fixed mass of gas is inversely proportional to the pressure, provided the temperature remains constant.
Coming up : How to measure the coefficient of expansion of a gas at constant pressure.