Why water does not expand like many substances?
 |
| iceberg-under-water |
Some substances do not always expand when heated. Over certain temperature ranges they contract. Water is an outstanding example.
If we take some water at 0° C and begin to heat it the water contracts instead of expanding over the temperature range 0 to 4° C.
Its behaviour is said to be anomalous. At about 4° C the water reaches its smallest volume, and this means that its density is now a maximum. If we continue to raise the temperature the water expands ( as you can see in the graph).
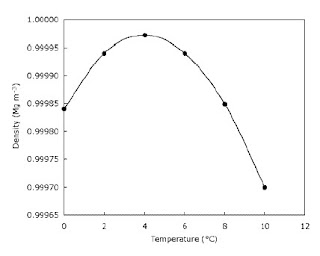 |
| Density of water changes with temperature |
compensated volume dilatometer
The volume changes described above my be demonstrated by means of a compensated volume dilatometer. ( the Fig. is in the previous post). This is a glass bulb with a stem attached and containing a quantity of mercury equal to one-seventh of the volume of the bulb.
A thermometer is fused into the bulb and rest of the space is filled up with water.
Why the quantity of mercury is one-seventh of the volume of the glass?
The answer is simple: The mercury compensates for the expansion of the glass. The coefficient of cubical expansion of the glass is about one-seventh that of mercury, and so any increase in volume of the glass bulb when it is heated will be exactly cancelled out by the equal expansion of the mercury inside it. The volume in the remaining space will, therefore, keep constant as the temperature changes.
Hence, if this remaining space is filled with water any movement of the water up the stem when the temperature is raised will represent the real and not the apparent expansion of the water.
The dilatometer is placed in melting ice and left until the water-level in the stem becomes steady. It is then taken out and allowed to warm up slowly while readings of temperature and water-level are taken at suitable intervals.
Between 0 and 4° C the level progressively falls showing a contraction in volume, but after this it begins to rise.
One disadvantage of the above method is the difficulty of ensuring uniformity of temperature throughout the water, since it is not possible to stir it.
Biological importance of the anomalous expansion of water
The peculiar expansion of water has an important bearing on the preservation of aquatic life during very cold weather.
 |
| water-cycle |
As the temperature of a pond or lake falls, the water contracts, becomes denser and sinks. A circulation is thus set up until all the water reaches its maximum density at 4° C.
In due course, ice forms on the top of the water, and after this the lower layers of water at 4° C can lose heat only by conduction.
Only very shallow water is thus liable to freeze solid. In deeper water there will always be water beneath the ice in which fish and other creatures can live (lucky fish!).
If you liked this post you can read it in a pdf format at the page named Download pdf.
