Energy budgets
An energy budget describes the ways in which energy is transformed from one state to another within some defined system, including an analysis of inputs, outputs, and changes in the quantities stored. Ecological energy budgets focus on the use and transformations of energy in the biosphere or its components.
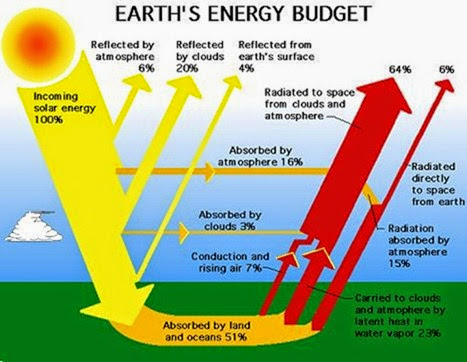
Solar electromagnetic radiation is the major input of energy to Earth. This external source of energy helps to heat the planet, evaporate water, circulate the atmosphere and oceans, and sustain ecological processes. Ultimately, all of the solar energy absorbed by Earth is re-radiated back to space, as electromagnetic radiation of a longer wavelength than what was originally absorbed. Earth maintains a virtually perfect energetic balance be- tween inputs and outputs of electromagnetic energy.
Earth’s ecosystems depend on solar radiation as an external source of diffuse energy that can be utilized by photosynthetic autotrophs, such as green plants, to synthesize simple organic molecules such as sugars from in- organic molecules such as carbon dioxide and water. Plants use the fixed energy of these simple organic com- pounds, plus inorganic nutrients, to synthesize an enormous diversity of biochemicals through various metabolic reactions. Plants utilize these biochemicals and the energy they contain to accomplish their growth and reproduction. Moreover, plant biomass is directly or indirectly utilized as food by the enormous numbers of heterotrophic organisms that are incapable of fixing their own energy. These organisms include herbivores that eat plants, carnivores that eat animals, and detritivores that feed on dead biomass.
Worldwide, the use of solar energy for this ecological purpose is relatively small, accounting for much less than 1% of the amount received at Earth’s surface. Al- though this is a quantitatively trivial part of Earth’s energy budget, it is clearly very important qualitatively, be- cause this is the absorbed and biologically fixed energy that subsidizes all ecological processes.
Forms of energy
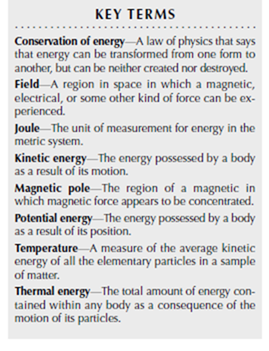
Energy is defined as the ability, or potential ability, of a body or system to do work. Energy can be measured in various units, such as the calorie, defined as the amount of energy required to raise the temperature of one gram of pure water from 59–61°F (15–16°C). (Note that the dietician’s calorie is equivalent to one thousand of these calories, or one kilocalorie.) The Joule is another unit of energy, defined as the amount of work required to lift a weight of 1 kg by 10 cm, and equivalent to 0.24 calories.
Energy can exist in various states, all of which are interchangeable through various sorts of physical/ chemical transformations. The basic categories of energy are: electromagnetic, kinetic, and potential, but each of these can also exist in various states, as is described below:
(1) Electromagnetic energy is the energy of photons, or quanta of energy that have properties of both particles and waves, and that travel through space at a constant speed of 3 X 108 meters per second (that is, at the speed of light). The components of electromagnetic energy are characterized on the basis of wavelength ranges, which ordered from the shortest to longest wavelengths are known as: gamma, x ray, ultraviolet, light or visible, infrared, and radio. All bodies with a temperature greater than absolute zero (that is, -459°F [-273°C], or zero degrees on the kelvin scale) emit electromagnetic energy at a rate and spectral quality that is strictly determined by their surface temperature. Relatively hot bodies have much larger emission rates and their radiation is dominated by shorter wavelengths, compared with cooler bodies. The Sun has a surface temperature of about 11,000°F (6,093°C) and most of its radiation is in the wavelength range of visible light (0.4-0.7 æm or micrometers) and shorter-wave infrared (0.7-2 æm), while Earth has a surface temperature of about 77°F (25°C) and its radiation peaks in the longer-wave infrared range at about 10 æm.
(2) Kinetic energy is the energy of dynamic motion, of which there are two basic types, the energy of moving bodies, and that of vibrating atoms or molecules. The later is also known as thermal energy, and the more vigorous the vibration, the greater the heat content.
(3) Potential energy has the capacity to do work, but it must be mobilized to do so. Potential energy occurs in various forms, including the following: (a) Chemical potential energy is stored in the inter-atomic bonds of molecules. This energy can be liberated by so-called exothermic reactions, which have a net release of energy. For example, heat is released when the chemically reduced sulfur of sulfide minerals is oxidized to sulfate, and when crystalline sodium chloride is dissolved into water. All biochemicals also store potential energy, equivalent to 4.6 kilocalories per gram of carbohydrate, 4.8 Kcal/g of protein, and 6.0-9.0 Kcal/g of fat. (b) Gravitational potential energy is stored in mass that is elevated above some gravitationally attractive surface, as when water occurs above the surface of the oceans, or any object occurs above the ground surface. Unless obstructed, water spontaneously flows downhill, and objects fall downwards in response to gradients of gravitational potential energy. (c) Other types of potential energy are somewhat less important in terms of ecological energy budgets, but they include potential energies of compressed gases, electrical potential gradients associated with voltage differentials, and the potential energy of matter, which can be released by nuclear reactions.
Energy transformations and the laws of thermodynamics
As noted previously, energy can be transformed among its various states. For example: (a) electromagnetic energy can be absorbed by a dark object and converted to thermal kinetic energy, resulting in an in- creased temperature of the absorbing body; (b) gravitational potential energy of water high on a plateau is transformed into the kinetic energy of moving water and heat at a waterfall, or it can be mobilized by humans to drive a turbine and generate electrical energy; and (c) solar electromagnetic radiation can be absorbed by the chlorophyll of green plants, and some of the absorbed energy can be converted into the chemical potential energy of sugars, and the rest converted into heat.
All transformations of energy must occur according to certain physical principles, known as the laws of thermodynamics. These are universal laws, which means that they are always true, regardless of circumstances. The first law states that energy can undergo transformations among its various states, but it is never created or destroyed, so the energy content of the universe remains constant. A consequence of this law for energy budgets is that there must always be a zero balance between the energy inputs to a system, the energy outputs, and any net storage within the system.
The second law of thermodynamics states that trans- formations of energy can only occur spontaneously under conditions in which there is an increase in the entropy of the universe. (Entropy is related to randomness of the distributions of matter and energy). For example, Earth is continuously irradiated by solar radiation, mostly of visible and near-infrared wavelengths. Some of this energy is absorbed, which heats the surface of Earth. The planet cools itself in various ways, but ultimately this is done by radiating its own electromagnetic radiation back to space, as longer- wave infrared radiation. The transformation of relatively short-wave solar radiation into the longer-wave radiation emitted by Earth represents a degradation of the quality of the energy, and an increase in the entropy of the universe.
A corollary, or secondary proposition of the second law of thermodynamics is that energy transformations can never be completely efficient, because some of the initial content of energy must be converted to heat so that entropy can be increased. Ultimately, this is the reason why no more than about 30% of the energy content of gasoline can be converted into the kinetic energy of a moving automobile, and why no more than about 40% of the energy of coal can be transformed into electricity in a modern generating station. Similarly, there are upper limits to the efficiency by which green plants can photo- synthetically convert visible radiation into biochemicals, even in ecosystems in which ecological constraints related to nutrients, water, and space are optimized.
Interestingly, plants absorb visible radiation emitted by the Sun, and use this relatively dispersed energy to fix simple inorganic molecules such as carbon dioxide, water, and other nutrients into very complex and energy- dense biochemicals. The biochemicals of plant biomass are then used by heterotrophic organisms to synthesize their own complex biochemicals. Locally, these various biological syntheses represent energy transformations that substantially decrease entropy, rather than increase it. This is because relatively dispersed solar energy and simple compounds are focused into the complex bio- chemicals of living organisms. Are biological transformations not obeying the second law of thermodynamics?
This seeming physical paradox of life can be successfully rationalized, using the following logic: The localized bio-concentrating of negative entropy can occur because there is a constant input of energy into the sys- tem, in the form of solar radiation. If this external source of energy was terminated, then all of the negative entropy of organisms and organic matter would rather quickly be spontaneously degraded, producing heat and simple inorganic molecules, and thereby increasing the entropy of the universe. This is why life and ecosystems cannot survive without continual inputs of solar energy. Therefore, the biosphere can be considered to represent a localized island, in space and time, of negative entropy, fueled by an external (solar) source of energy. There are physical analogues to these ecological circumstances—if external energy is put into the system, relatively dispersed molecules of gases can be concentrated into a container, as occurs when a person blows energetically to fill a balloon with air. Eventually, however, the balloon pops, the gases re-disperse, the original energy input is converted into heat, and the entropy of the universe is increased.